Question 90 of 96 How many moles of NH, form when 32.4 Lof H gas completely reacts at STP according to the following reaction? Remember 1 mol of an ideal gas has a volume of 22.4 L at STP Na(9) - 3 Ha(g)- 2 NH,(@) STARTING AMOUNT ADO FACTOR ANSMER RESET 0.964 3 32.4 6.022 x 10" 2.02 1.45 4.34 2 22.4 0.482 16.4 17.03 mol H, omol H, LH; mol NH, 9 NH, g/mol NH, L NH, 8 主
Question 90 of 96 How many moles of NH, form when 32.4 Lof H gas completely reacts at STP according to the following reaction? Remember 1 mol of an ideal gas has a volume of 22.4 L at STP Na(9) - 3 Ha(g)- 2 NH,(@) STARTING AMOUNT ADO FACTOR ANSMER RESET 0.964 3 32.4 6.022 x 10" 2.02 1.45 4.34 2 22.4 0.482 16.4 17.03 mol H, omol H, LH; mol NH, 9 NH, g/mol NH, L NH, 8 主
Chapter2: Loads On Structures
Section: Chapter Questions
Problem 1P
Related questions
Question
Please help me with this conversion factors with lables on it. Please show me the work.

Transcribed Image Text:Question 90 of 96
How many moles of NH, form when 32.4 Lof H gas completely reacts at STP
according to the following reaction? Remember 1 mol of an ideal gas has a volume
of 22.4 L at STP
Na(9) - 3 Ha(g)-
2 NH,(@)
STARTING AMOUNT
ADO FACTOR
ANSMER
RESET
0.964
3
32.4
6.022 x 10"
2.02
1.45
4.34
2
22.4
0.482
16.4
17.03
mol H, omol H,
LH;
mol NH,
9 NH,
g/mol NH,
L NH,
8 主
Expert Solution
Step 1
At STP,
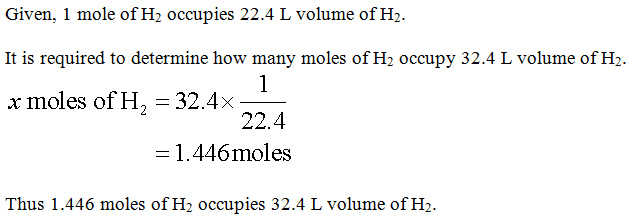
Step 2
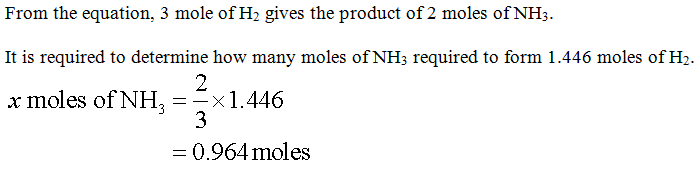
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, civil-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you


Structural Analysis (10th Edition)
Civil Engineering
ISBN:
9780134610672
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Principles of Foundation Engineering (MindTap Cou…
Civil Engineering
ISBN:
9781337705028
Author:
Braja M. Das, Nagaratnam Sivakugan
Publisher:
Cengage Learning


Structural Analysis (10th Edition)
Civil Engineering
ISBN:
9780134610672
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Principles of Foundation Engineering (MindTap Cou…
Civil Engineering
ISBN:
9781337705028
Author:
Braja M. Das, Nagaratnam Sivakugan
Publisher:
Cengage Learning

Fundamentals of Structural Analysis
Civil Engineering
ISBN:
9780073398006
Author:
Kenneth M. Leet Emeritus, Chia-Ming Uang, Joel Lanning
Publisher:
McGraw-Hill Education


Traffic and Highway Engineering
Civil Engineering
ISBN:
9781305156241
Author:
Garber, Nicholas J.
Publisher:
Cengage Learning