Question 4.) a. I2(s) is_________ in NH3(l) Choices: soluble, miscible, immiscible, insoluble b. C3H8(l) is________ in NH3(l) Choices: soluble, miscible, immiscible, insoluble c. PCl3(l) is________ in NH3(l) Choices: soluble, miscible, immiscible, insoluble d. PBr3(s) is___________ in NH3(l) Choices: soluble, miscible, immiscible, insoluble
Question 4.) a. I2(s) is_________ in NH3(l) Choices: soluble, miscible, immiscible, insoluble b. C3H8(l) is________ in NH3(l) Choices: soluble, miscible, immiscible, insoluble c. PCl3(l) is________ in NH3(l) Choices: soluble, miscible, immiscible, insoluble d. PBr3(s) is___________ in NH3(l) Choices: soluble, miscible, immiscible, insoluble
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 12.50PAE: In Exercise 12.49, what is the allowed concentration of AgCl in g per 100 g of water? The Safe...
Related questions
Question
Question 4.)
a. I2(s) is_________ in NH3(l)
Choices: soluble, miscible, immiscible, insoluble
b. C3H8(l) is________ in NH3(l)
Choices: soluble, miscible, immiscible, insoluble
c. PCl3(l) is________ in NH3(l)
Choices: soluble, miscible, immiscible, insoluble
d. PBr3(s) is___________ in NH3(l)
Choices: soluble, miscible, immiscible, insoluble
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
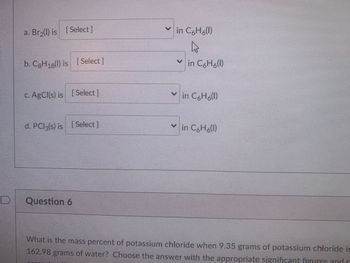
Transcribed Image Text:### Solubility in Benzene (C₆H₆)
1. **a. Br₂(l)**:
- Choose the appropriate solubility status from the dropdown selection for Br₂ (liquid) in benzene (C₆H₆).
2. **b. C₈H₁₈(l)**:
- Choose the appropriate solubility status from the dropdown selection for C₈H₁₈ (liquid) in benzene (C₆H₆).
3. **c. AgCl(s)**:
- Choose the appropriate solubility status from the dropdown selection for AgCl (solid) in benzene (C₆H₆).
4. **d. PCl₃(s)**:
- Choose the appropriate solubility status from the dropdown selection for PCl₃ (solid) in benzene (C₆H₆).
---
### Question 6
**Problem Statement:**
What is the mass percent of potassium chloride when 9.35 grams of potassium chloride is dissolved in 162.98 grams of water? Choose the answer with the appropriate significant figures and enter your answer.
Solution
Follow-up Question
![**Transcription for Educational Website:**
### Solubility Exercise
Choose the appropriate solubility state for each compound in benzene (C₆H₆(l)):
a. Br₂(l) is [Select ▼] in C₆H₆(l)
b. C₈H₁₈(l) is [Select ▼] in C₆H₆(l)
c. AgCl(s) is [Select ▼] in C₆H₆(l)
d. PCl₃(s) is [Select ▼] in C₆H₆(l)
### Instructions:
Use the dropdown menu to select whether each compound is soluble or insoluble in benzene. Consider the polarity and molecular structure when determining the solubility.](https://content.bartleby.com/qna-images/question/d41439c0-1722-4f03-91aa-8380bbf7ac66/24f7d470-ce10-4e66-9eab-5d8bb4dd12ac/j1gpw7p_thumbnail.jpeg)
Transcribed Image Text:**Transcription for Educational Website:**
### Solubility Exercise
Choose the appropriate solubility state for each compound in benzene (C₆H₆(l)):
a. Br₂(l) is [Select ▼] in C₆H₆(l)
b. C₈H₁₈(l) is [Select ▼] in C₆H₆(l)
c. AgCl(s) is [Select ▼] in C₆H₆(l)
d. PCl₃(s) is [Select ▼] in C₆H₆(l)
### Instructions:
Use the dropdown menu to select whether each compound is soluble or insoluble in benzene. Consider the polarity and molecular structure when determining the solubility.
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

