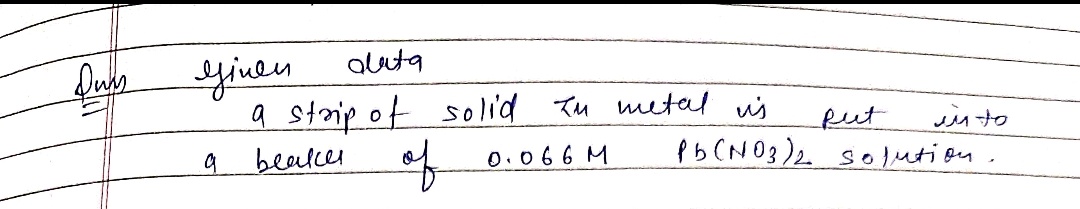
Question 1 Objective Knowledge Check Decide whether a chemical reaction happens in either of the following situations. If a reaction does happen, write the chemical equation for it. Be sure your chemical equation is balanced and has physical state symbols. chemical situation chemical equation reaction? A strip of solid zinc metal is put into a beaker of 0.066M Pb(NO3)2 solution. O yes O no A strip of solid lead metal is put into a beaker of 0.078M Zn(NO,)2 solution. O yes O no
Question 1 Objective Knowledge Check Decide whether a chemical reaction happens in either of the following situations. If a reaction does happen, write the chemical equation for it. Be sure your chemical equation is balanced and has physical state symbols. chemical situation chemical equation reaction? A strip of solid zinc metal is put into a beaker of 0.066M Pb(NO3)2 solution. O yes O no A strip of solid lead metal is put into a beaker of 0.078M Zn(NO,)2 solution. O yes O no
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:Question 1
Ca
11
Objective Knowledge Check
Decide whether a chemical reaction happens in either of the following situations. If a reaction does happen, write the chemical equation for it. Be sure your
chemical equation is balanced and has physical state symbols.
chemical
situation
chemical equation
reaction?
A strip of solid zinc metal
is put into a beaker of
0.066M Pb(NO3)2 solution.
O yes
O no
A strip of solid lead metal
is put into a beaker of
0.078M Zn(NO3)2 solution.
О yes
O no
I Don't Know
Submit
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY