Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
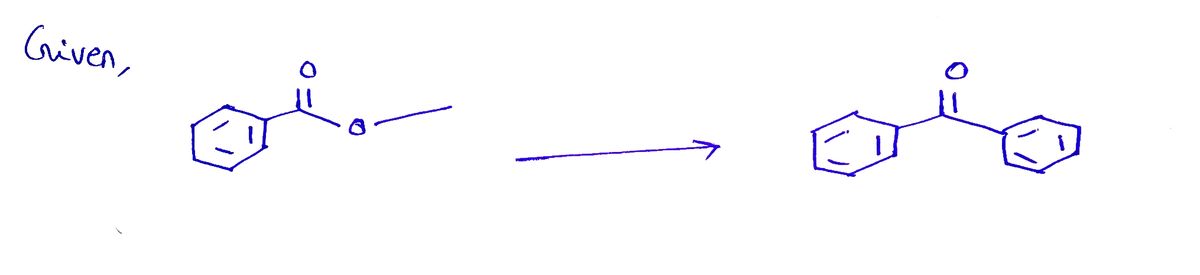
I have proposed a suitable synthesis for the following transformation please let me know whether or not this is correct. If incorrect please let me know what I did wrong.

Transcribed Image Text:**Title: Propose a Suitable Synthesis**
**Header:** Synthesis Steps
**Text and Diagram Explanation:**
1. **Reactants and Reagents:**
- The synthesis begins with a benzene ring attached to a carbon chain with a protected alcohol group.
- The first reaction shows the use of lithium aluminum hydride (LiAlH₄) followed by hydronium ion (H₃O⁺), which suggests a reduction step.
2. **Formation of Brominated Product:**
- The second step involves PBr₃, which is typically used to convert alcohols to alkyl bromides, indicating the conversion of a hydroxyl group to a bromine atom on a benzene ring.
3. **Grignard Reagent Formation:**
- The next step involves magnesium in dry ether, converting the alkyl bromide to a Grignard reagent (R-MgBr).
4. **Grignard Reaction with Carbonyl:**
- In a separate reaction, a ketone group is treated with the Grignard reagent, forming a tertiary alcohol. The conditions indicated are:
1) Reaction of the carbonyl compound with Grignard reagent.
2) Protonation with H₃O⁺ to yield the final product.
5. **Oxidation:**
- The tertiary alcohol product goes through oxidation with PCC (pyridinium chlorochromate) to form a ketone structure.
**Summary:**
This process illustrates the transformation of a benzene-derived alcohol through a series of reductions, brominations, Grignard reactions, and oxidations to synthesize a benzophenone derivative. The mechanisms and reagents are indicative of fundamental organic synthesis techniques used to manipulate functional groups and carbon backbones effectively.
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY