Problem1 olteoililqmia bns anoliqmnA obolM soiiqm Jud oo s lenoijoon 22010 odi oz leohoda ai l ovel no abrog6 (novigal bvol bos omuly biupil noswY noi Stream 1 rigu vol 22sm tolo ban tolnl ny(t), Ibmoles/min T1(t), °F x(t), mole fraction of A upiladeve Stream 3 P(D, psia nz(t), Ibmoles/min T3(t), °F YA(t), mole fraction of AM P3, psia Stream 2 Pure A nzlt), Ibmoles/min T2(t), °F n(t), Ibmoles moto 200mled cusLEA
Problem1 olteoililqmia bns anoliqmnA obolM soiiqm Jud oo s lenoijoon 22010 odi oz leohoda ai l ovel no abrog6 (novigal bvol bos omuly biupil noswY noi Stream 1 rigu vol 22sm tolo ban tolnl ny(t), Ibmoles/min T1(t), °F x(t), mole fraction of A upiladeve Stream 3 P(D, psia nz(t), Ibmoles/min T3(t), °F YA(t), mole fraction of AM P3, psia Stream 2 Pure A nzlt), Ibmoles/min T2(t), °F n(t), Ibmoles moto 200mled cusLEA
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
100%
Can you answer this Question For me . Do Dynamic modeling ((Mixing of gas stream).in this picture

Transcribed Image Text:Problem1
olteoililqmia bns anoliqmnA obolM
soiiqm
Jud oo s lenoijoon 22010 odi oz leohoda ai l
ovel no abrog6
(novigal
bvol bos omuly biupil noswY
noi
Stream 1
rigu vol 22sm tolo ban tolnl
ny(t), Ibmoles/min
T1(t), °F
x(t), mole fraction
of A
upiladeve
Stream 3
P(D, psia
nz(t), Ibmoles/min
T3(t), °F
YA(t), mole fraction
of AM
P3, psia
Stream 2
Pure A
nzlt), Ibmoles/min
T2(t), °F
n(t),
Ibmoles
moto
200mled
cusLEA
Expert Solution
Step 1
Assume that the gas obeys the ideal gas law. Also, the specific heat capacity of all the streams is taken as constants.
Let, Cp1, Cp2, and Cp3 be the specific heat capacities of streams 1, 2, and 3 respectively.
State variables for this system are taken as T3, yA, ṅ3, and P3.
Step 2
Apply overall mole balance on the given system as:
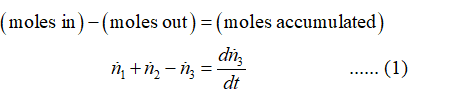
Step 3
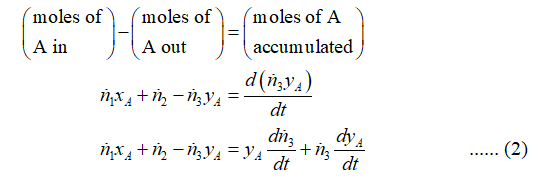
Step by step
Solved in 6 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The