Part A CaO can be used as a drying agent. One such application occurs when water is added to dry concrete or cement. The reaction that occurs is CaO (s) + H₂O(1) Ca(OH)2 (s) The product is commonly called slaked lime. Assuming the commonly used standard-state temperature of 25°C, calculate A.Stotal for this reaction using table from the table below. μA ΔΗ So [J/(K-mol)] (kJ/mol) CaO(s) 39.9 -635.1 H₂O(1) 69.9 -285.8 Ca(OH)2 (s) 83.4 -986.1 Substance Express your answer to three significant figures and include the appropriate units. To indicate multiplication in a compound unit, use a multiplication dot (e.g., K. mol). ▸ View Available Hint(s) ?
Part A CaO can be used as a drying agent. One such application occurs when water is added to dry concrete or cement. The reaction that occurs is CaO (s) + H₂O(1) Ca(OH)2 (s) The product is commonly called slaked lime. Assuming the commonly used standard-state temperature of 25°C, calculate A.Stotal for this reaction using table from the table below. μA ΔΗ So [J/(K-mol)] (kJ/mol) CaO(s) 39.9 -635.1 H₂O(1) 69.9 -285.8 Ca(OH)2 (s) 83.4 -986.1 Substance Express your answer to three significant figures and include the appropriate units. To indicate multiplication in a compound unit, use a multiplication dot (e.g., K. mol). ▸ View Available Hint(s) ?
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question
3L3.1
![According to the second law of thermodynamics, all
reactions proceed spontaneously in the direction
that increases total entropy. The total entropy is
defined as
AStotal = AS system + AS surroundings
The direction of a spontaneous change is always
determined by the sign of the total entropy change:
• The reaction is spontaneous if
AStotal > 0.
• The reaction is nonspontaneous if
AStotal < 0.
• The reaction is at equilibrium if
AStotal = 0.
The value of AStotal depends on AS system and
AS surroundings, which are defined by
AS system = So (products) – S° (reactants)
-
and
AS surroundings
-AH system
T
Spontaneity is therefore affected by the enthalpy of
the system and the Kelvin temperature.
Part A
CaO can be used as a drying agent. One such application occurs when water is added to dry concrete
or cement. The reaction that occurs is
The product is commonly called slaked lime.
Assuming the commonly used standard-state temperature of 25°C, calculate AStotal for this reaction
using table from the table below.
CaO (s) + H₂O(1) = Ca(OH)2 (s)
μᾶ
Value
Submit
Substance
Express your answer to three significant figures and include the appropriate units. To indicate
multiplication in a compound unit, use a multiplication dot (e.g., K. mol).
View Available Hint(s)
So
ΔΗ
[J/(K·mol)] (kJ/mol)
39.9
-635.1
-285.8
-986.1
CaO(s)
H₂O(1)
69.9
Ca(OH)2 (s) 83.4
Units
?](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F6484a2ba-7b6c-4144-bd9b-1f2d784a131b%2F822b55f4-8ab4-42b4-9c3a-62a462a37d28%2Fg4ivi6k_processed.jpeg&w=3840&q=75)
Transcribed Image Text:According to the second law of thermodynamics, all
reactions proceed spontaneously in the direction
that increases total entropy. The total entropy is
defined as
AStotal = AS system + AS surroundings
The direction of a spontaneous change is always
determined by the sign of the total entropy change:
• The reaction is spontaneous if
AStotal > 0.
• The reaction is nonspontaneous if
AStotal < 0.
• The reaction is at equilibrium if
AStotal = 0.
The value of AStotal depends on AS system and
AS surroundings, which are defined by
AS system = So (products) – S° (reactants)
-
and
AS surroundings
-AH system
T
Spontaneity is therefore affected by the enthalpy of
the system and the Kelvin temperature.
Part A
CaO can be used as a drying agent. One such application occurs when water is added to dry concrete
or cement. The reaction that occurs is
The product is commonly called slaked lime.
Assuming the commonly used standard-state temperature of 25°C, calculate AStotal for this reaction
using table from the table below.
CaO (s) + H₂O(1) = Ca(OH)2 (s)
μᾶ
Value
Submit
Substance
Express your answer to three significant figures and include the appropriate units. To indicate
multiplication in a compound unit, use a multiplication dot (e.g., K. mol).
View Available Hint(s)
So
ΔΗ
[J/(K·mol)] (kJ/mol)
39.9
-635.1
-285.8
-986.1
CaO(s)
H₂O(1)
69.9
Ca(OH)2 (s) 83.4
Units
?
Expert Solution
Step 1: Calculation for ∆Hsystem
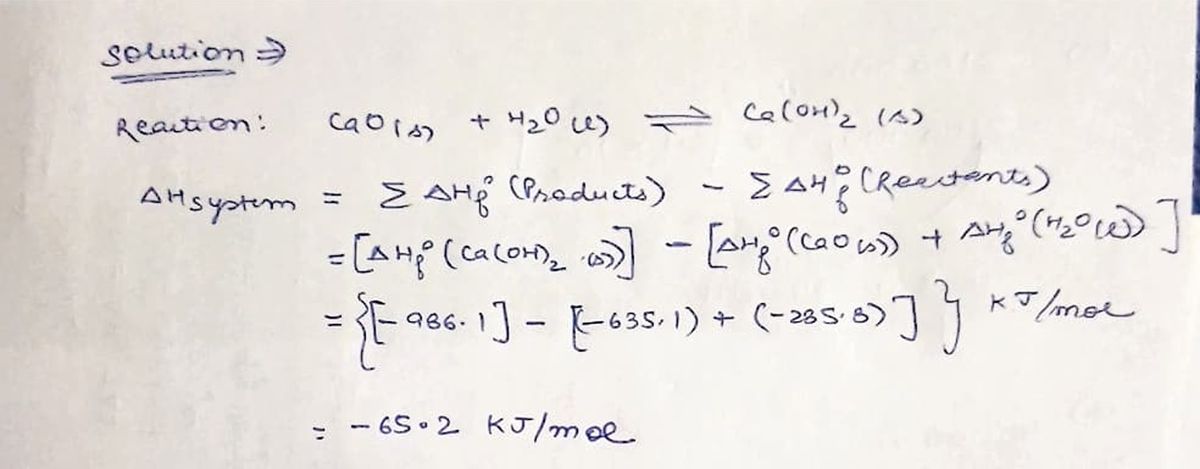
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON