Page 8 of 35 Your Learning Points 4 Osiagwu, Chiemeka K Iron metal reacts with elemental sulfur to produce iron (III) sulfide. What mass of iron is required to react with 13. 61 g of sulfur?
Page 8 of 35 Your Learning Points 4 Osiagwu, Chiemeka K Iron metal reacts with elemental sulfur to produce iron (III) sulfide. What mass of iron is required to react with 13. 61 g of sulfur?
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:O (81) Drake - In The Bible (Official x
Answered: Drag the text element X
Chapter 3 Competency 5
G molar mass of fe - Google Search x
+
->
A cloud.scorm.com/ScormEnginelnterface/defaultui/player/legacy.html?configuration=CbMtix7R173giv8Jlq2qNte5-N4X7iO1ARnDq98KvBHVj4XKlo1rLn9XYyotbblQjPsc2h... *
I Apps © Google MyMasteryPath
co Welcome to LSU M.. P MasteringBiology E FRENCH
ALEKS – Adaptive L.
Pt Periodic Table - Pta.
Other bookmarks
E Reading list
Chapter 3 Competency 5
POWERED BY THE RUSTICI EN
PREVIOUS
NEXT
CLOSE ITEM
RETURN TO LMS
Page 8 of 35
Your Learning Points 4
Osiagwu, Chiemeka K
Iron metal reacts with elemental sulfur to produce iron (III) sulfide. What mass of iron is
required to react with 13. 61 g of sulfur?
(A) 79. 99 g
Check your
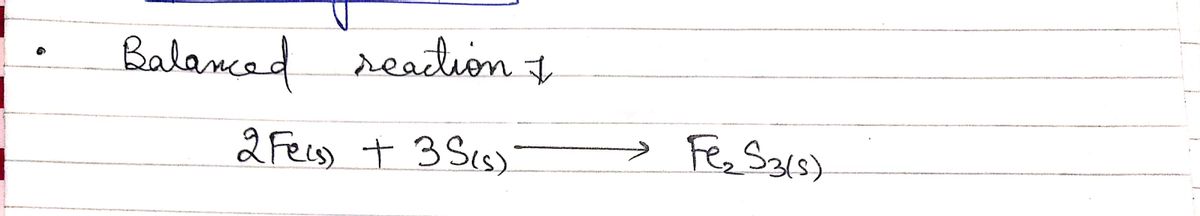
balanced reaction
2 Fe(s) + 3 S(s) -> Fe,S, (s)
(В) 53. 33 g
(C) 61. 33 g
(D) 15. 8 g
(E) 35. 55 g
9:27 PM
O Type here to search
A 4)
9/25/2021
1O
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY