College Physics
11th Edition
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Raymond A. Serway, Chris Vuille
Chapter1: Units, Trigonometry. And Vectors
Section: Chapter Questions
Problem 1CQ: Estimate the order of magnitude of the length, in meters, of each of the following; (a) a mouse, (b)...
Related questions
Question
Find the work done during one cycle of the process shown in the diagram below.

Transcribed Image Text:The image displays a pressure-volume (P-V) diagram, an essential tool in thermodynamics used to illustrate the changes in pressure and volume for a closed system. The axes are labeled as follows:
- The vertical axis (y-axis) represents pressure, \( P \), measured in atmospheres (atm).
- The horizontal axis (x-axis) represents volume, \( V \), measured in cubic meters (m\(^3\)).
The diagram shows a triangular cycle traced by the orange arrows, indicating a thermodynamic process involving three distinct stages:
1. **Starting from the leftmost point**: The process moves horizontally from a volume of approximately 1.00 m\(^3\) to 2.00 m\(^3\) while maintaining a constant pressure of around 6.00 atm.
2. **Second leg of the cycle**: The process descends diagonally from around 6.00 atm to 2.00 atm, with the volume increasing slightly above 1.00 m\(^3\).
3. **Final leg of the cycle**: The path ascends vertically, returning to the initial pressure of approximately 6.00 atm while the volume decreases back to about 1.00 m\(^3\).
This cyclic process forms a closed loop, implying that the system undergoes a series of transformations and eventually returns to its initial state. The direction of the arrows suggests the sequence of the process stages.
Expert Solution
Step 1
Given:-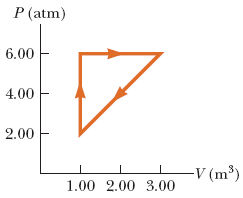
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

University Physics (14th Edition)
Physics
ISBN:
9780133969290
Author:
Hugh D. Young, Roger A. Freedman
Publisher:
PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:
9781107189638
Author:
Griffiths, David J., Schroeter, Darrell F.
Publisher:
Cambridge University Press

College Physics
Physics
ISBN:
9781305952300
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning

University Physics (14th Edition)
Physics
ISBN:
9780133969290
Author:
Hugh D. Young, Roger A. Freedman
Publisher:
PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:
9781107189638
Author:
Griffiths, David J., Schroeter, Darrell F.
Publisher:
Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:
9781337553278
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:
9780321820464
Author:
Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:
Addison-Wesley

College Physics: A Strategic Approach (4th Editio…
Physics
ISBN:
9780134609034
Author:
Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:
PEARSON