OPO,2- \OH HO myo-inositol-1-phosphate synthase OH 2-03PO HO "OH HO (1 ÕH a-D-glucose-6-phosphate myo-inositol-1-phosphate a. What is the absolute configuration (R or S) at C-2 of naturally occurring a-D-glucose-6-phosphate? b. Using any unambiguous notation, draw the enantiomer (mirror image) of myo- inositol-1-phosphate. Place your answer in the space provided here.
OPO,2- \OH HO myo-inositol-1-phosphate synthase OH 2-03PO HO "OH HO (1 ÕH a-D-glucose-6-phosphate myo-inositol-1-phosphate a. What is the absolute configuration (R or S) at C-2 of naturally occurring a-D-glucose-6-phosphate? b. Using any unambiguous notation, draw the enantiomer (mirror image) of myo- inositol-1-phosphate. Place your answer in the space provided here.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Myo-inositol, the most prominent naturally occurring form of inositols, is a carbocyclic polyol that plays an important role as the structural basis for a number of secondary messengers in eukaryotic cells. It is generated in vivo from the aldol cyclization of glucose-6-phosphate to myo-inositol-1-phosphate

Transcribed Image Text:**Transcription and Explanation for Educational Website**
---
**Chemical Transformations and Stereochemistry:**
The image illustrates a biochemical reaction facilitated by the enzyme **myo-inositol-1-phosphate synthase**. Two chemical structures are involved in this transformation:
1. **α-D-glucose-6-phosphate** on the left.
2. **myo-inositol-1-phosphate** on the right.
The structures are depicted with their respective phosphate groups, hydroxyl groups, and carbon atom configurations.
**Key Features:**
- **α-D-glucose-6-phosphate:** Exhibits a ring structure common to glucose derivatives, with multiple hydroxyl (-OH) groups and a phosphate group (2^O3PO) attached at the sixth carbon. The chiral center at the second carbon (C-2) is of particular interest here.
- **myo-inositol-1-phosphate:** A cyclic alcohol with a phosphate group attached. This molecule results from the enzyme-catalyzed conversion of α-D-glucose-6-phosphate.
**Questions for Consideration:**
a. **Absolute Configuration Analysis:**
- The diagram prompts the identification of the absolute configuration (R or S) at the C-2 position of naturally occurring α-D-glucose-6-phosphate.
b. **Enantiomer Drawing:**
- The diagram requests the drawing of the enantiomer (mirror image) of myo-inositol-1-phosphate. The space provided next to this task implies the expectation for a detailed, accurate depiction using any unambiguous notation.
---
**Graphs and Diagrams Explanation:**
- The arrows between the two chemical structures indicate the two-way enzymatic conversion between α-D-glucose-6-phosphate and myo-inositol-1-phosphate.
- The stereochemistry around each chiral center is marked using wedges and dashes to denote the three-dimensional orientation of attached groups.
This image and accompanying questions are typical in studies focusing on metabolic pathways and the stereochemical implications of enzymatic processes.
Expert Solution
Step 1
Absolute configuration is R. This can be calculated based on the CIP rule. 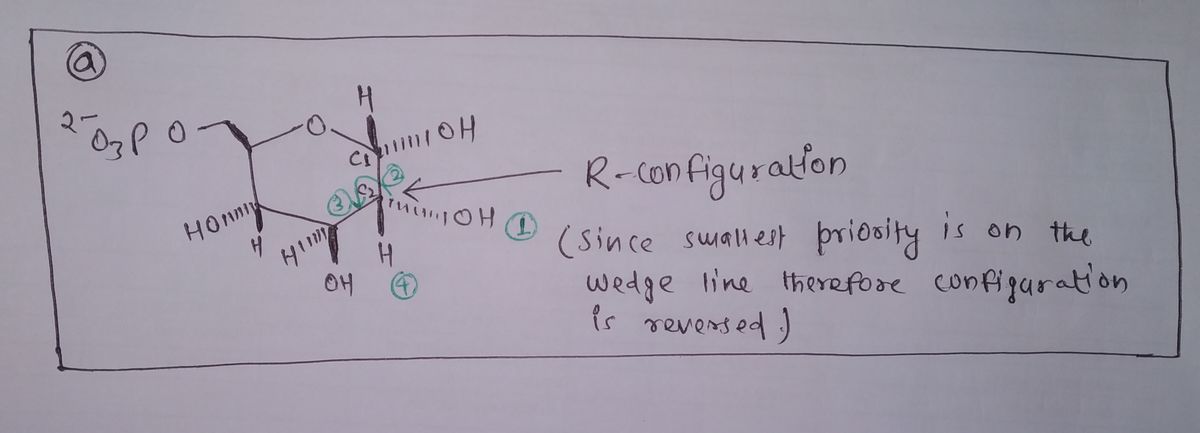
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY