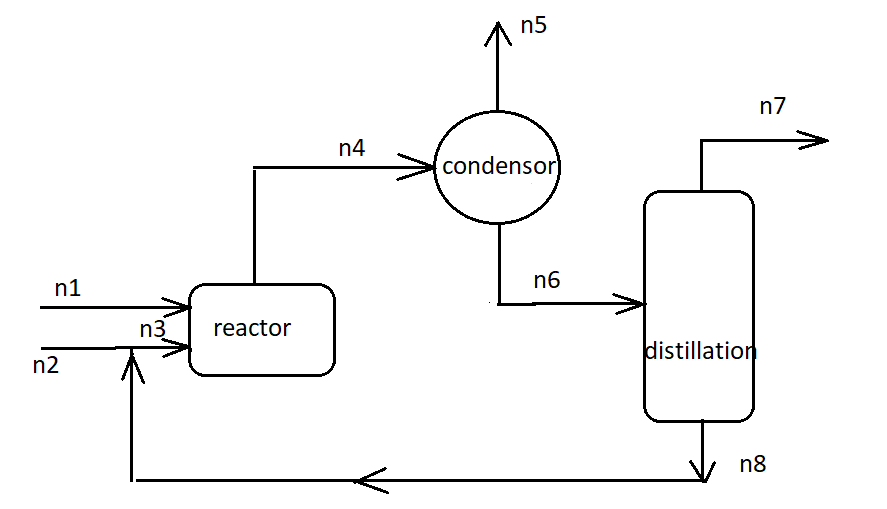
One of the ways that benzene is produced on a large scale is the hydrodealkylation of toluene. CH₂CH3 + H₂ ->>> • CoHo +CH_ A stream of toluene is mixed with a recycle stream and enters a reactor along with a stream of pure hydrogen. The reaction products at 550 °C enter a condenser, where they are cooled to 43.0 °C. A vapor stream containing YSCH, = 0.530 mol CH₂/mol leaves the process, and a liquid stream containing x6b = 0.740 mol benzene/mol and x6 = 0.260 mol toluene/mol enters a distillation column. The distillate of the column leaves the process at n = 781.0 mol/h and contains y7b=0.9300 mol benzene/mol and y7= 0.0700 mol toluene/mol. The bottoms of the column contains X8b = 0.240 mol benzene/mol and x8t = 0.760 mol toluene/mol and is recycled back to the fresh feed. Hydrogen is fed into the process at n₁ = 1710 mol H₂/h. This process is carried out at 760 mmHg.
One of the ways that benzene is produced on a large scale is the hydrodealkylation of toluene. CH₂CH3 + H₂ ->>> • CoHo +CH_ A stream of toluene is mixed with a recycle stream and enters a reactor along with a stream of pure hydrogen. The reaction products at 550 °C enter a condenser, where they are cooled to 43.0 °C. A vapor stream containing YSCH, = 0.530 mol CH₂/mol leaves the process, and a liquid stream containing x6b = 0.740 mol benzene/mol and x6 = 0.260 mol toluene/mol enters a distillation column. The distillate of the column leaves the process at n = 781.0 mol/h and contains y7b=0.9300 mol benzene/mol and y7= 0.0700 mol toluene/mol. The bottoms of the column contains X8b = 0.240 mol benzene/mol and x8t = 0.760 mol toluene/mol and is recycled back to the fresh feed. Hydrogen is fed into the process at n₁ = 1710 mol H₂/h. This process is carried out at 760 mmHg.
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
Please post the steps!
![**Hydrodealkylation of Toluene for Benzene Production**
One of the ways that benzene is produced on a large scale is through the hydrodealkylation of toluene. The chemical reaction involved is:
\[ \text{C}_6\text{H}_5\text{CH}_3 + \text{H}_2 \rightarrow \text{C}_6\text{H}_6 + \text{CH}_4 \]
A stream of toluene is combined with a recycle stream and introduced into a reactor along with a stream of pure hydrogen. The reaction occurs at 550°C. The products then enter a condenser where they are cooled to 43.0°C.
- The vapor stream contains:
- \( y_{\text{CH}_4} = 0.530 \) mol CH₄/mol
This vapor stream exits the process, and a liquid stream, with the following composition, enters a distillation column:
- \( x_{6\text{B}} = 0.740 \) mol benzene/mol
- \( x_{6\text{T}} = 0.260 \) mol toluene/mol
The distillate from the column exits the process at \( n_7 = 781.0 \) mol/h with:
- \( y_{7\text{B}} = 0.930 \) mol benzene/mol
- \( y_{7\text{T}} = 0.070 \) mol toluene/mol
The column bottoms contain:
- \( x_{8\text{B}} = 0.240 \) mol benzene/mol
- \( x_{8\text{T}} = 0.760 \) mol toluene/mol
The bottom stream is then recycled back to the fresh feed. Hydrogen is fed into the process at \( n_1 = 1710 \) mol H₂/h. This entire process is conducted at a pressure of 760 mmHg.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fe8e4ba9c-f4e2-4406-a528-e4a92bd942c6%2F9d00044c-0db1-4c6d-8a13-c405aa2c43ec%2F3dhgd59_processed.jpeg&w=3840&q=75)
Transcribed Image Text:**Hydrodealkylation of Toluene for Benzene Production**
One of the ways that benzene is produced on a large scale is through the hydrodealkylation of toluene. The chemical reaction involved is:
\[ \text{C}_6\text{H}_5\text{CH}_3 + \text{H}_2 \rightarrow \text{C}_6\text{H}_6 + \text{CH}_4 \]
A stream of toluene is combined with a recycle stream and introduced into a reactor along with a stream of pure hydrogen. The reaction occurs at 550°C. The products then enter a condenser where they are cooled to 43.0°C.
- The vapor stream contains:
- \( y_{\text{CH}_4} = 0.530 \) mol CH₄/mol
This vapor stream exits the process, and a liquid stream, with the following composition, enters a distillation column:
- \( x_{6\text{B}} = 0.740 \) mol benzene/mol
- \( x_{6\text{T}} = 0.260 \) mol toluene/mol
The distillate from the column exits the process at \( n_7 = 781.0 \) mol/h with:
- \( y_{7\text{B}} = 0.930 \) mol benzene/mol
- \( y_{7\text{T}} = 0.070 \) mol toluene/mol
The column bottoms contain:
- \( x_{8\text{B}} = 0.240 \) mol benzene/mol
- \( x_{8\text{T}} = 0.760 \) mol toluene/mol
The bottom stream is then recycled back to the fresh feed. Hydrogen is fed into the process at \( n_1 = 1710 \) mol H₂/h. This entire process is conducted at a pressure of 760 mmHg.

Transcribed Image Text:**Educational Content:**
**Determining Mole Fractions and Flow Rates in a Vapor Stream**
This exercise focuses on calculating the mole fractions of benzene and toluene in a vapor stream, as well as determining the flow rates in a chemical process.
**Parameters:**
- **\( y_{5b} \):** Mole fraction of benzene (b) in the vapor stream leaving the condenser.
- Unit: mol b/mol
- **\( y_{5t} \):** Mole fraction of toluene (t) in the vapor stream leaving the condenser.
- Unit: mol t/mol
- **\( n_2 \):** Flow rate of fresh toluene into the process.
- Unit: mol t/hr
- **\( n_8 \):** Flow rate of the recycle stream.
- Unit: mol/hr
**Exercise:**
1. Calculate \( y_{5b} \) using the given information or a provided formula.
2. Calculate \( y_{5t} \) using the given information or a provided formula.
3. Determine \( n_2 \) which is the input flow rate of fresh toluene.
4. Calculate \( n_8 \) which represents the flow rate of the recycle stream in the process.
Complete the calculations and input your results in the respective boxes for each parameter. This exercise helps in understanding the distribution and flow rates of chemical compounds in industrial processes.
Expert Solution
Step 1
Write the reaction as follows:
Draw the flow diagram according to the given problem as follows:
Step by step
Solved in 6 steps with 1 images

Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The