Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Draw the Newman projections for the most and least stable conformations and indicate what strain(s) the least stable conformation has. Look in the direction of arrow.

Transcribed Image Text:This is the structural formula for (R)-3-Fluoro-3-methyl-2-butanol, an organic compound.
### Explanation:
- **Carbon Backbone**: The structure includes a four-carbon chain. The main chain appears in a zigzag line format, which is typical for organic line structures.
- **Functional Groups**:
- The hydroxyl group (OH) is attached to the second carbon in the chain.
- There is a fluorine atom (F) attached to the third carbon in the chain.
- **Stereochemistry**:
- The structure includes wedge-and-dash notation to indicate stereochemistry.
- The solid wedge (pointing towards the viewer) indicates that the group (a CH3 group) is coming out of the plane of the page.
- The dashed line (pointing away from the viewer) indicates that the fluorine atom is going into the plane of the page.
- This configuration suggests that it is the R-enantiomer of 3-fluoro-3-methyl-2-butanol.
- **Branches**:
- There is a methyl group (CH3) attached to the third carbon, supporting the complexity of the structure.
This compound can have various applications and relevance in organic chemistry, studying enantiomers, and understanding stereochemistry.
Expert Solution
Step 1
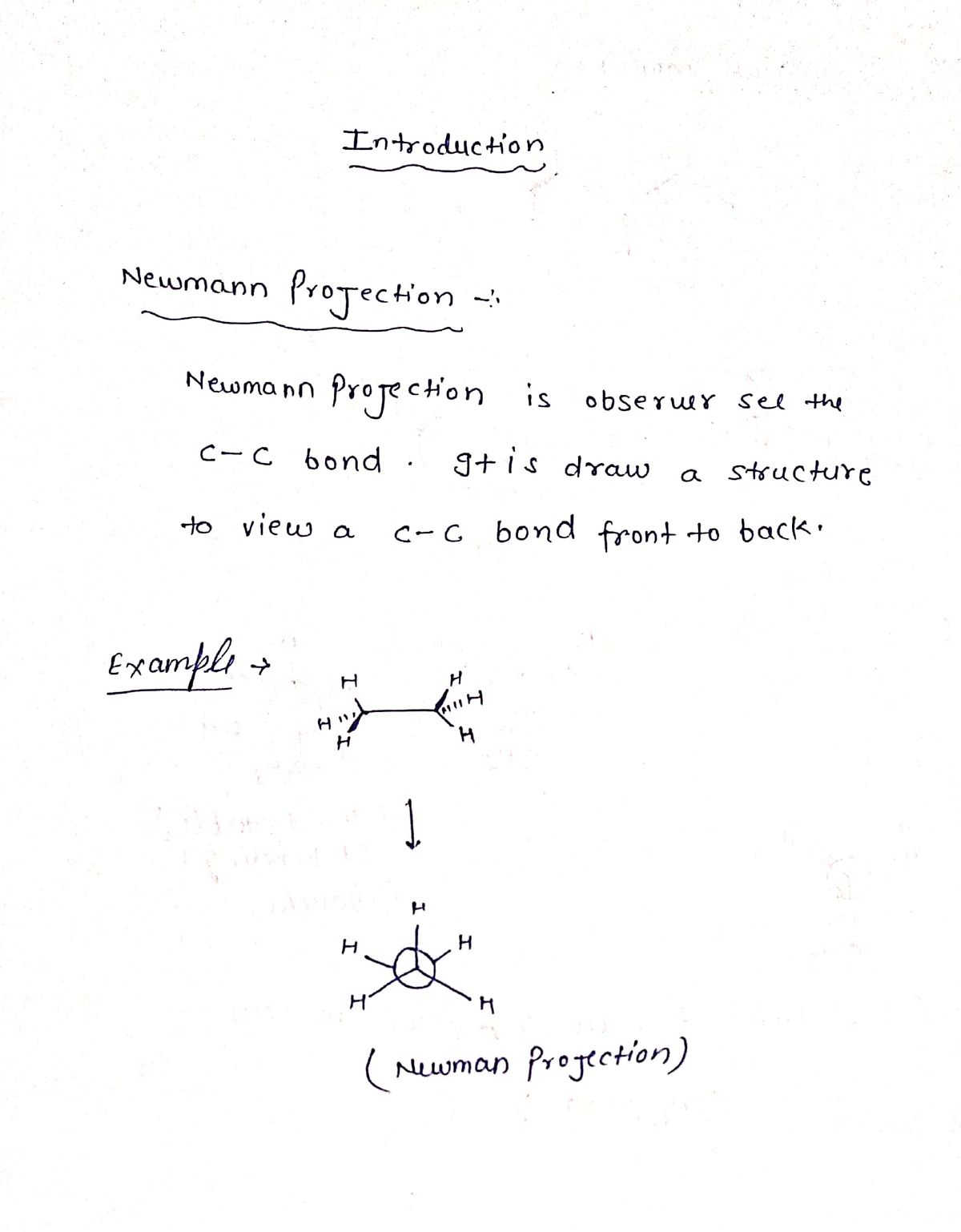
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY