Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
![**Transcription for Educational Website:**
---
**Problem Statement:**
\[[\text{OH}^-] = 4.2 \times 10^{-2}\]
Express the concentration to two significant figures and include the appropriate units.
---
**Input Section:**
- A text box labeled with \[[\text{H}_3\text{O}^+]\] is provided for entering the answer.
- The input is divided into two fields: "Value" and "Units".
**Toolbar:**
- Contains various icons for formatting and editing:
- An icon for options that resemble layout adjustments.
- A "μÅ" icon, possibly related to formatting.
- Undo and redo arrows.
- A refresh icon.
- A keyboard icon for text input.
- A question mark for help or additional information.
---
When filling in the input fields, ensure the concentration is expressed in scientific notation with the correct units. Use the toolbar options as needed to assist with formatting and input.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F8bccc44d-7782-46f2-838e-c140c2dedd7b%2F0060c45a-61a3-4468-8173-9c993e9b3820%2F33jfb0g_processed.png&w=3840&q=75)
Transcribed Image Text:**Transcription for Educational Website:**
---
**Problem Statement:**
\[[\text{OH}^-] = 4.2 \times 10^{-2}\]
Express the concentration to two significant figures and include the appropriate units.
---
**Input Section:**
- A text box labeled with \[[\text{H}_3\text{O}^+]\] is provided for entering the answer.
- The input is divided into two fields: "Value" and "Units".
**Toolbar:**
- Contains various icons for formatting and editing:
- An icon for options that resemble layout adjustments.
- A "μÅ" icon, possibly related to formatting.
- Undo and redo arrows.
- A refresh icon.
- A keyboard icon for text input.
- A question mark for help or additional information.
---
When filling in the input fields, ensure the concentration is expressed in scientific notation with the correct units. Use the toolbar options as needed to assist with formatting and input.
![You may want to reference (Page) Section 16.5 while completing this problem.
Calculate \([ \text{H}_3\text{O}^+ ]\) at 25°C for each solution and determine whether the solution is acidic, basic, or neutral.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F8bccc44d-7782-46f2-838e-c140c2dedd7b%2F0060c45a-61a3-4468-8173-9c993e9b3820%2F2s9jy4_processed.png&w=3840&q=75)
Transcribed Image Text:You may want to reference (Page) Section 16.5 while completing this problem.
Calculate \([ \text{H}_3\text{O}^+ ]\) at 25°C for each solution and determine whether the solution is acidic, basic, or neutral.
Expert Solution
Step 1
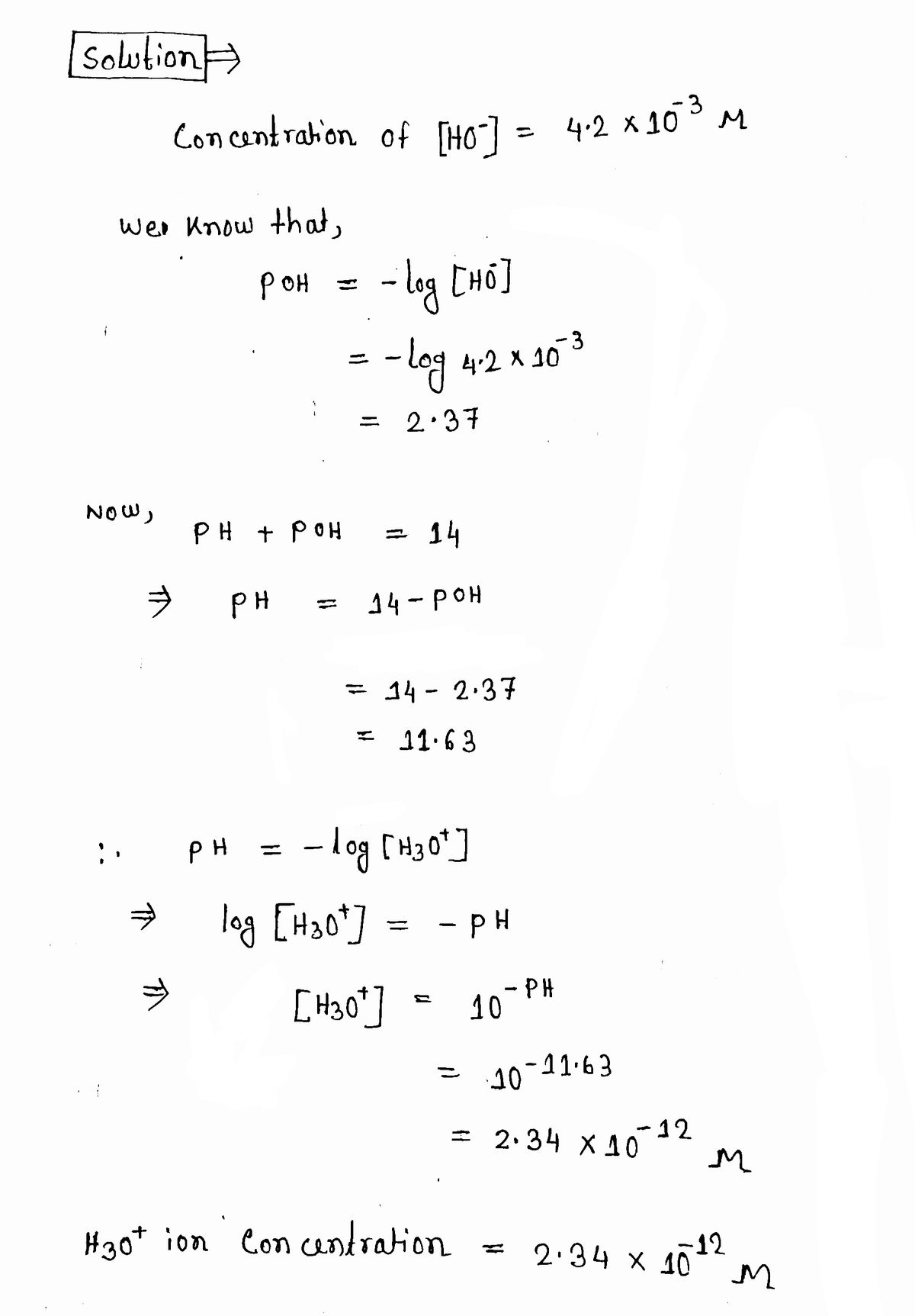
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY