Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter12: Chemical Equilibrium
Section: Chapter Questions
Problem 113QRT
Related questions
Question
![**Educational Content: Understanding Chemical Equilibrium Changes**
In this exercise, we explore how the concentration of each species in a chemical reaction changes to reestablish equilibrium after alterations in the system. The reaction under consideration is:
\[ \text{H}_2(g) + \text{Br}_2(g) \rightleftharpoons 2\text{HBr}(g) \]
**Instructions**:
- Use the arrows to determine the change in concentration for each species:
- An **up arrow** (↑) signifies an increase in concentration.
- A **down arrow** (↓) indicates a decrease in concentration.
- Leaving it blank means no change in concentration.
**Scenario Analysis**:
1. If the concentration of H₂ is decreased:
- \[ \text{H}_2: \quad \downarrow \]
- \[ \text{Br}_2: \quad \uparrow \]
- \[ \text{HBr}: \quad \downarrow \]
2. If the concentration of HBr is increased:
- \[ \text{H}_2: \quad \uparrow \]
- \[ \text{Br}_2: \quad \uparrow \]
- \[ \text{HBr}: \quad \downarrow \]
**Answer Bank**:
The representation uses boxes below the molecular formulas and allows for the selection of the appropriate arrows based on reaction changes, guiding students through the process of visualizing equilibrium shifts in concentrations.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2Fc5c0fdfc-ed48-4a05-a6ae-d17833402f74%2Ff57b4916-9811-4c1b-a06a-204e874cbb18%2Fmn91qxh.jpeg&w=3840&q=75)
Transcribed Image Text:**Educational Content: Understanding Chemical Equilibrium Changes**
In this exercise, we explore how the concentration of each species in a chemical reaction changes to reestablish equilibrium after alterations in the system. The reaction under consideration is:
\[ \text{H}_2(g) + \text{Br}_2(g) \rightleftharpoons 2\text{HBr}(g) \]
**Instructions**:
- Use the arrows to determine the change in concentration for each species:
- An **up arrow** (↑) signifies an increase in concentration.
- A **down arrow** (↓) indicates a decrease in concentration.
- Leaving it blank means no change in concentration.
**Scenario Analysis**:
1. If the concentration of H₂ is decreased:
- \[ \text{H}_2: \quad \downarrow \]
- \[ \text{Br}_2: \quad \uparrow \]
- \[ \text{HBr}: \quad \downarrow \]
2. If the concentration of HBr is increased:
- \[ \text{H}_2: \quad \uparrow \]
- \[ \text{Br}_2: \quad \uparrow \]
- \[ \text{HBr}: \quad \downarrow \]
**Answer Bank**:
The representation uses boxes below the molecular formulas and allows for the selection of the appropriate arrows based on reaction changes, guiding students through the process of visualizing equilibrium shifts in concentrations.
Expert Solution
Step 1
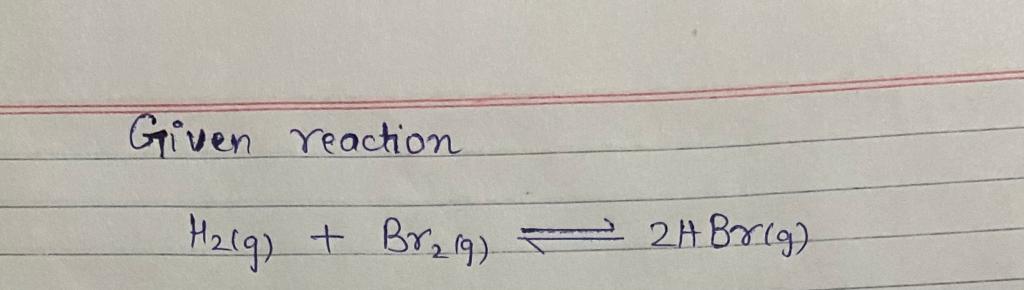
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co