NOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part. An adiabatic air compressor compresses 10.4 L/s of air at 120 kPa and 20°C to 1000 kPa and 300°C. The constant pressure specific heat of air at the average temperature of 160°C = 433 K is cp= 1.018 kJ/kg-K. The gas constant of air is R = 0.287 kPa.m³/kg.K. 1 MPa 300°C Compressor 120 kPa 20°C VUS Determine the work required by the compressor. (You must provide an answer before moving on to the next part.) The work required by the compressor is -4.578 kJ/kg.
NOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part. An adiabatic air compressor compresses 10.4 L/s of air at 120 kPa and 20°C to 1000 kPa and 300°C. The constant pressure specific heat of air at the average temperature of 160°C = 433 K is cp= 1.018 kJ/kg-K. The gas constant of air is R = 0.287 kPa.m³/kg.K. 1 MPa 300°C Compressor 120 kPa 20°C VUS Determine the work required by the compressor. (You must provide an answer before moving on to the next part.) The work required by the compressor is -4.578 kJ/kg.
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question

Transcribed Image Text:### Educational Content: Adiabatic Air Compressor Analysis
#### Problem Description:
This is a multi-part question. Once an answer is submitted, you will be unable to return to this part.
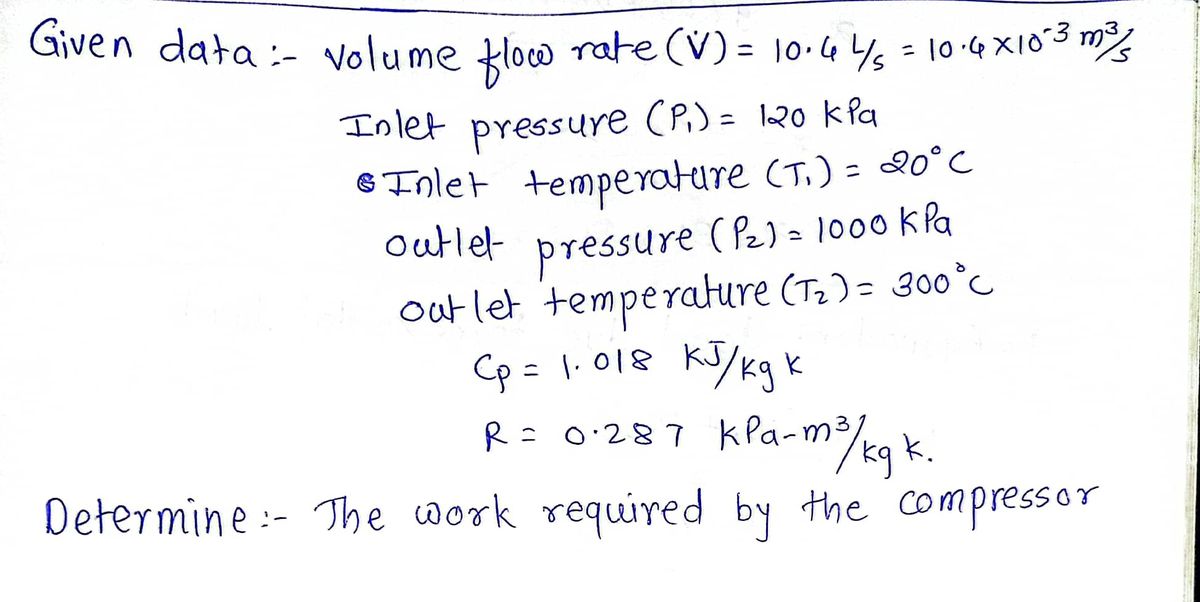
An adiabatic air compressor compresses 10.4 L/s of air at 120 kPa and 20°C to 1000 kPa and 300°C. The constant pressure specific heat of air at the average temperature of 160°C = 433 K is \( c_p = 1.018 \, \text{kJ/kg.K} \). The gas constant of air is \( R = 0.287 \, \text{kPa} \cdot \text{m}^3/\text{kg.K} \).
#### Diagram Explanation:
- The diagram shows a compressor.
- Air enters the compressor at:
- 120 kPa
- 20°C
- 10.4 L/s (volumetric flow rate)
- Air exits the compressor at:
- 1 MPa (1000 kPa)
- 300°C
#### Task:
Determine the work required by the compressor. *(You must provide an answer before moving on to the next part.)*
#### Solution:
The work required by the compressor is \( -4.578 \, \text{kJ/kg} \).
Expert Solution
Step 1: Determine the given variable
Step by step
Solved in 3 steps with 3 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY