
Lewis structure represents the systematic arrangement of atoms around the central atom. Electrons in this structure are indicated by dots. The central atom is the least electronegative element in a molecule.
The given molecule is . Oxygen (O) is more electronegative than nitrogen (N). Therefore, N is the central atom in the molecule.
The N atom has 5 valence electrons and O has 6 valence electrons. Calculate the total number of valence electrons in .
The total number of valence electrons in are 18. The number of valence electron pairs is half of the total number of valence electrons. Therefore, the number of valence electron pairs in are 9, which is 18/2.
Draw the skeletal structure of by assigning 2 valence electron pairs between N and O atoms (two on each side of N).
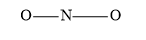
The remaining valence electron pairs are assigned as the lone pairs to the surrounding two O atoms. In this way, 4 electron pairs are used. With the distribution of electron pairs the octet of one O is completed.
The central N atom does not have a complete octet of electrons. Therefore, one pair of electrons remain as lone pair on the nitrogen atom. Another pair forms a double bond with the oxygen atom.
Draw the complete Lewis dot structure of .
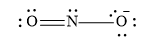
Step by step
Solved in 5 steps with 2 images









