Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Can you help with the Last one thats highlighted please?

Transcribed Image Text:**Title: Understanding Nucleophilic Substitution Reactions: SN1 and SN2**
**Introduction**
In organic chemistry, nucleophilic substitution reactions are fundamental processes where a nucleophile displaces a leaving group attached to a carbon atom. The two main types are SN1 and SN2 reactions. This guide explains how to determine the appropriate reactants and solvent systems for synthesizing a series of compounds via substitution reactions.
**Step-by-Step Analysis**
**Compound 1:**
- Starting Material: Alkyl bromide
- Reaction: NaCN in acetone leads to the substitution of bromine with a cyano group.
- Type: SN2
- Explanation: The reactant is a primary halide, and there is an inversion of configuration, indicating an SN2 reaction mechanism.
**Compound 2:**
- Starting Material: Cyclohexyl bromide
- Reaction: Ammonia (NH3) replaces the bromine with an amino group.
- Type: SN1
- Explanation: The presence of a tertiary halide and racemization suggest an SN1 mechanism. The carbocation intermediate allows for the reformation of bonds and loss of stereochemistry.
**Compound 3:**
- Starting Material: Tertiary alkyl bromide
- Reaction: Conversion to an alcohol using HBr.
- Type: SN1
- Explanation: The tertiary halide structure fosters an SN1 reaction pathway due to the stability of the carbocation intermediate.
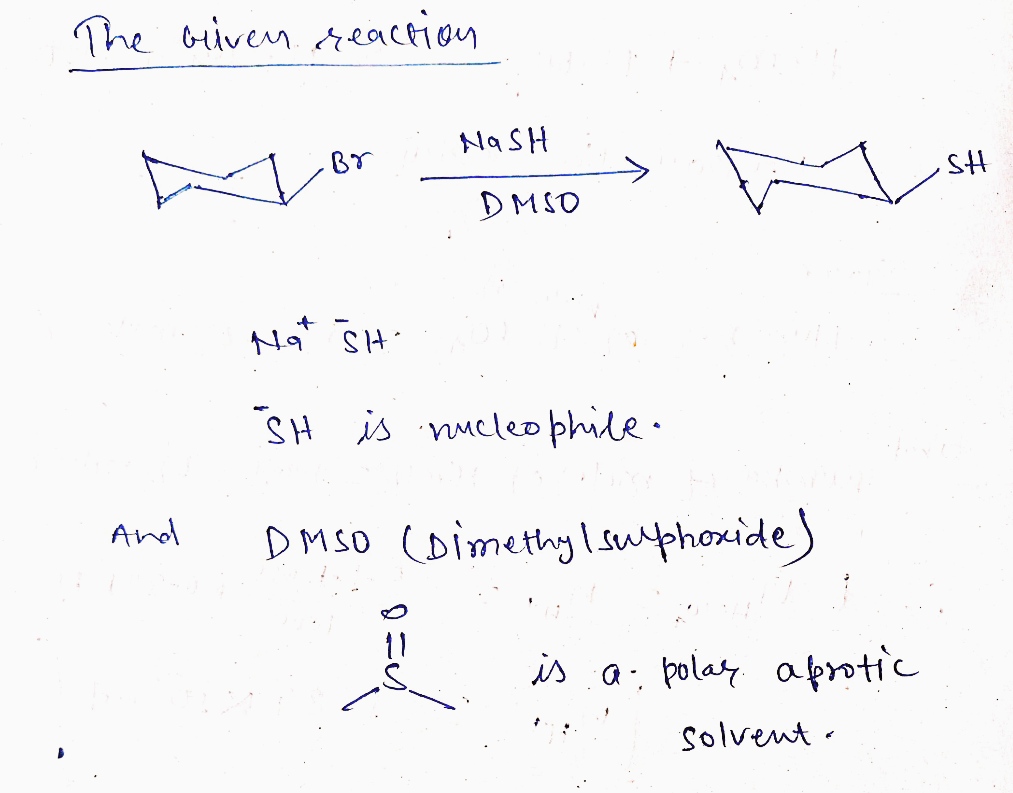
**Compound 4:**
- Starting Material: Cyclohexyl bromide
- Reaction: Sodium hydrosulfide (NaSH) in DMSO replaces the bromine with a thiol group.
- Type: SN1
- Further Inquiry Required: The diagram is highlighted with "Help" and a question mark, indicating uncertainty or need for further clarification on the specifics of this reaction.
**Conclusion**
By analyzing the structure of the starting material and the conditions provided, one can determine the mechanism (SN1 or SN2) of nucleophilic substitution. Understanding these details is crucial for predicting product formation and optimizing reaction conditions in synthetic organic chemistry.
Expert Solution
Step 1
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY