Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:Select the missing reagents in the following multistep synthesis. Ignore any inorganic
byproducts formed.
'H
Š
H
O
H₂O
OH
@
1. NaOH, heat
2. Neutralizing work-up
1
H3O+
O
TSOH, H₂O
O
H
a
A
B
C
D
E
HOCH2CH2CH₂OH
NaOH
HOCH2CH2CH₂OH
TSOH
HOCH2CH2CH₂OH
H₂O
TSOH, H₂O
CH₂OH(2 eq)
TSOH

Transcribed Image Text:O
H₂O
OH
O
OH
| a
Q
2. Neutralizing work-up
H3O+
TSOH, H₂O
O
A
B
C
D
E
mCPBA
H3O+
1. BH3-THF
2.H₂O2, NaOH
Br₂
H₂O
1. OsO4 (catalytic)
2. NMO
Expert Solution
Step 1: Explanation
Carbonyl compounds having alpha-H undergo condensation in presence of a base to form alpha, beta-unsaturated carbonyl compounds.
Diols acts as a protecting group for Carbonyl compounds.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
The last reaction is either C, D, or E.
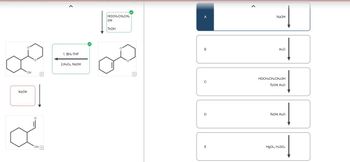
Transcribed Image Text:### Educational Website Content: Chemistry Reaction Pathway
#### Reaction Schemes
1. **Cyclohexene to Cyclohexanol Oxidation Reaction:**
- **Reactants:** Cyclohexene
- **Reagents:**
- 1. BH₃·THF
- 2. H₂O₂, NaOH
- **Product:** Cyclohexanol
- **Description:** This two-step reaction involves the hydroboration of cyclohexene followed by oxidation to convert the alkene into an alcohol.
2. **Conversion of Alcohol to Ketone:**
- **Reactants:** Cyclohexanol
- **Reagent:** NaOH
- **Product:** Cyclohexanone
- **Description:** This step oxidizes the alcohol group into a ketone.
#### Flowchart of Chemical Transformations
- **Path A:**
- Starting material reacts with NaOH.
- The expected product from this step is not specified, but is part of a sequence of transformations.
- **Path B:**
- Subsequent step with H₂O addition.
- **Path C:**
- Reaction with HOCH₂CH₂CH₂OH and TsOH, followed by TsOH, H₂O.
- **Path D:**
- Direct reaction with TsOH, H₂O.
- **Path E:**
- Reaction involving HgCl₂ and H₂SO₄.
#### Diagram Explanation
The diagram on the left illustrates chemical structures and reaction arrows, indicating the sequence of transformations from cyclohexene to cyclohexanone through intermediate steps involving hydroboration/oxidation, followed by further conversion. The right side lists potential pathways and reagents that might be used in extended reaction schemes, showing a logical sequence of chemical changes leading to the final products.
This reaction overview highlights fundamental organic chemistry processes, showcasing the transformation of starting materials through various reagents to achieve target molecules used in synthetic strategies.
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY