molecules to produce 16 carbon dioxide molecules and 18 water molecules. Use of fossil fuels adds CO2, carbon dioxide, to the atmosphere. This human-generated
Gasoline is primarily composed of iso-octane, which has the chemical formula C8H18. When gasoline is burned, the chemical equation is:
2C8H18 + 25O2 ---> 16CO2 + 18H2O
This means that 2 molecules of iso-octane combine with 25 oxygen molecules to produce 16 carbon dioxide molecules and 18 water molecules. Use of fossil fuels adds CO2, carbon dioxide, to the atmosphere. This human-generated CO2 increases the planetary greenhouse effect, leading to an increase in average land surface temperatures, accumulation of heat in the oceans, and modification of global climate. Part of the problem is that a lot of CO2 is produced per mass of fuel burned. You can see that by looking at the fraction of carbon in iso-octane and carbon dioxide.
1. Calculate the formula masses of iso-octane (C8H18) and carbon dioxide (CO2)
2. What is the percent composition of carbon (C) in C8H18 and CO2?
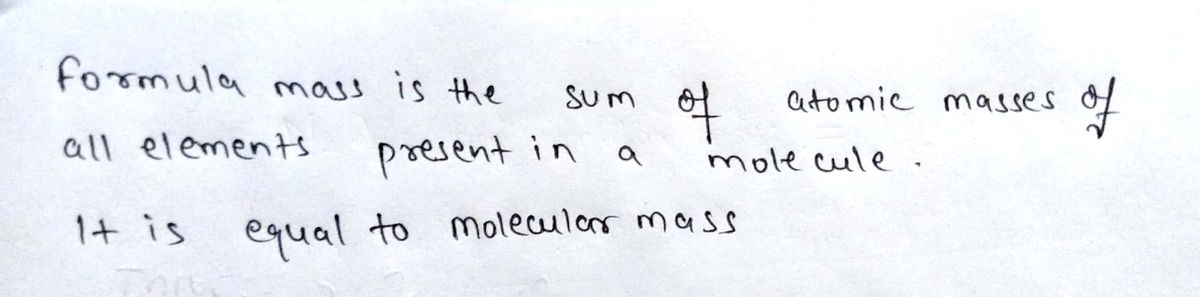
Step by step
Solved in 2 steps with 2 images









