Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
I want the one on the down right one to be answered thank you. Was confused do we have a CHO around 2800cm-1

Transcribed Image Text:# Infrared Spectra Analysis of Isomers of C₄H₈O
## Overview
Below are the infrared spectra for four isomers of C₄H₈O. Each spectrum features characteristic peaks that help in identifying functional groups present in the compounds. The task is to propose a structure for each isomer based on their IR spectra.
## Infrared Spectra Descriptions
### Spectrum 1
- **Wavenumber Range:** 4000 - 500 cm⁻¹
- **Key Peaks:**
- ~2900 cm⁻¹: C-H stretching
- ~1720 cm⁻¹: C=O stretching, indicative of a carbonyl group
- ~2700 cm⁻¹: Aldehyde C-H bond
### Spectrum 2
- **Wavenumber Range:** 4000 - 500 cm⁻¹
- **Key Peaks:**
- Broad peak at ~3300 cm⁻¹: O-H stretch, indicative of alcohol
- ~2900 cm⁻¹: C-H stretching
- ~1100 cm⁻¹: C-O stretching
### Spectrum 3
- **Wavenumber Range:** 4000 - 500 cm⁻¹
- **Key Peaks:**
- ~2250 cm⁻¹: C≡C stretch, indicating a triple bond
- Broad peak at ~3300 cm⁻¹: Possible O-H stretch
### Spectrum 4
- **Wavenumber Range:** 4000 - 500 cm⁻¹
- **Key Peaks:**
- ~1640 cm⁻¹: C=C stretch, suggesting an alkene
- ~2900 cm⁻¹: C-H stretching
## Proposed Structures
- **First Spectrum**: Suggests an aldehyde due to C=O and aldehyde C-H peaks.
- **Second Spectrum**: Indicates an alcohol due to the broad O-H stretch.
- **Third Spectrum**: Points to a terminal alkyne or alcohol with triple bond C≡C.
- **Fourth Spectrum**: Consistent with an alkene due to the C=C stretch.
Each spectrum analysis focuses on functional group identification to aid in determining the correct isomeric structure of C₄H₈O.
Expert Solution
Step 1
Given molecular formula is C4H8O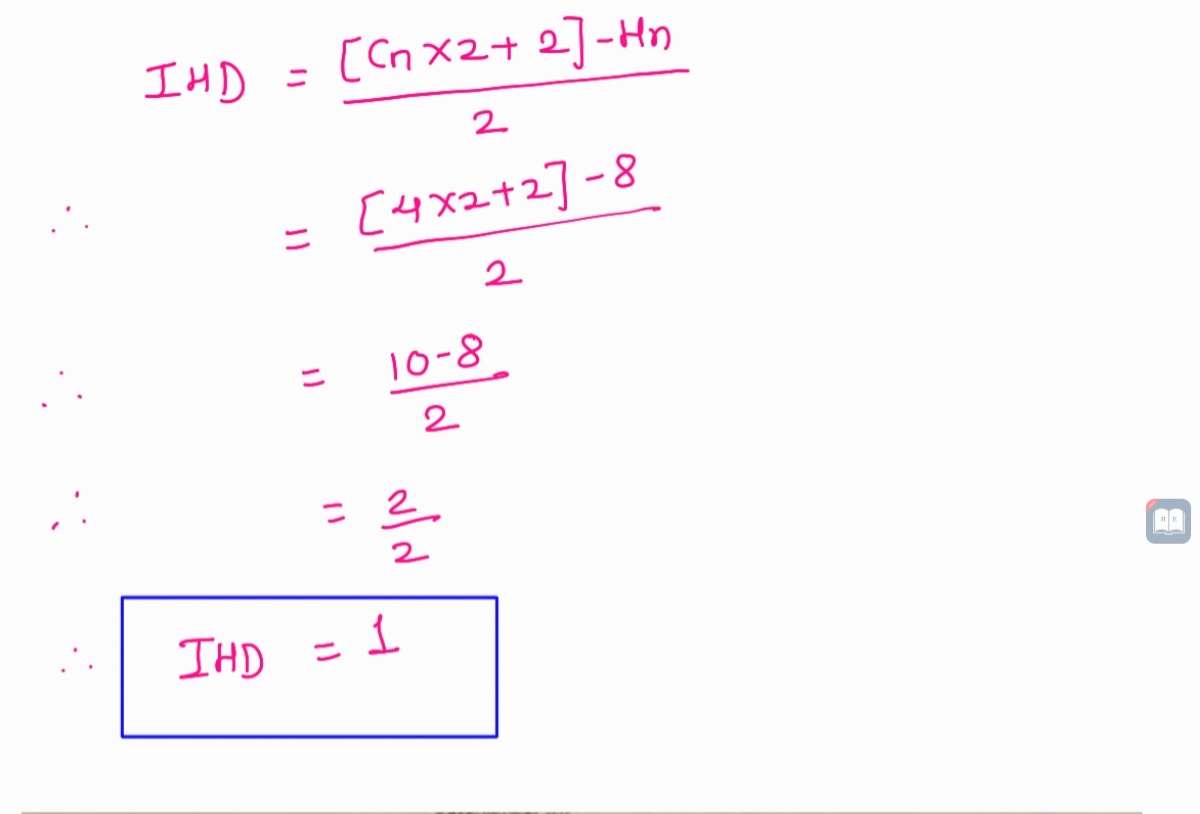
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY