mass of solvent, kg ) 4. Determine the value for the van't Hoff factor (i) for each of your solutes. For molecular solutes the value is i = 1. For ionic solutes, you can determine the value of i by examining the molecular formula. The value will be a small whole number; partial ionization is not expected, and waters of hydration are not included.
mass of solvent, kg ) 4. Determine the value for the van't Hoff factor (i) for each of your solutes. For molecular solutes the value is i = 1. For ionic solutes, you can determine the value of i by examining the molecular formula. The value will be a small whole number; partial ionization is not expected, and waters of hydration are not included.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
I need help with question 4.
The initial temperature for all 3 is 0.9 degrees C.

Transcribed Image Text:Calculations
1. Using the solute formulas provided, convert grams of solute to moles of solute. For the hydrated
salts be sure to include the waters of hydration in the molar mass.
2. It is reasonable to assume that the total mass of solvent includes the mass of both the water
and the ice. If you used exactly 50.0 g of each, then you would have used 100.0 g or 0.100 kg of
solvent. Use the actual masses of water and ice recorded in your lab notebook to calculate the
mass of solvent.
3. Calculate the molality of the solution using the moles of solute (from Step 1) and the mass of
solvent (from Step 2):
moles of solute
mass of solvent, kg
molality, m =
4. Determine the value for the van't Hoff factor (i) for each of your solutes. For molecular solutes
the value is i = 1. For ionic solutes, you can determine the value of i by examining the molecular
formula. The value will be a small whole number; partial ionization is not expected, and waters
of hydration are not included.
5. Calculate the theoretical freezing point depression for each of the solutions in °C. The k, for
water is 1.86°C/m.
6. Calculate the theoretical freezing point of each solution, and compare it with the
experimentally observed freezing point for your solutes.
7. Comment in your lab notebook about the relationship between the observed freezing points
and the number of particles in solution (i) for each solute.
14
Colligative Pr o perties of Solutions- Freezing Point Depression | Labor atory 2

Transcribed Image Text:Molality
i
Theoretical
Mass of
Mass of
Mass of
Observed
Solute
solute (g)
ice (g)
water (g)
f.p. (°C)
(m)
f.p. (°C)
2.91 4894 49.92-1°C 049
NaCl
CaCl, · 2H;O 7.37 5643 51.700.0c 013
17.12 A85 51.17
NA
C12H2„O11
0.క 043
5°C
Fe(NO3)3 · 9H2O|NA
NA
NA
NA NA NA
Expert Solution
Step 1
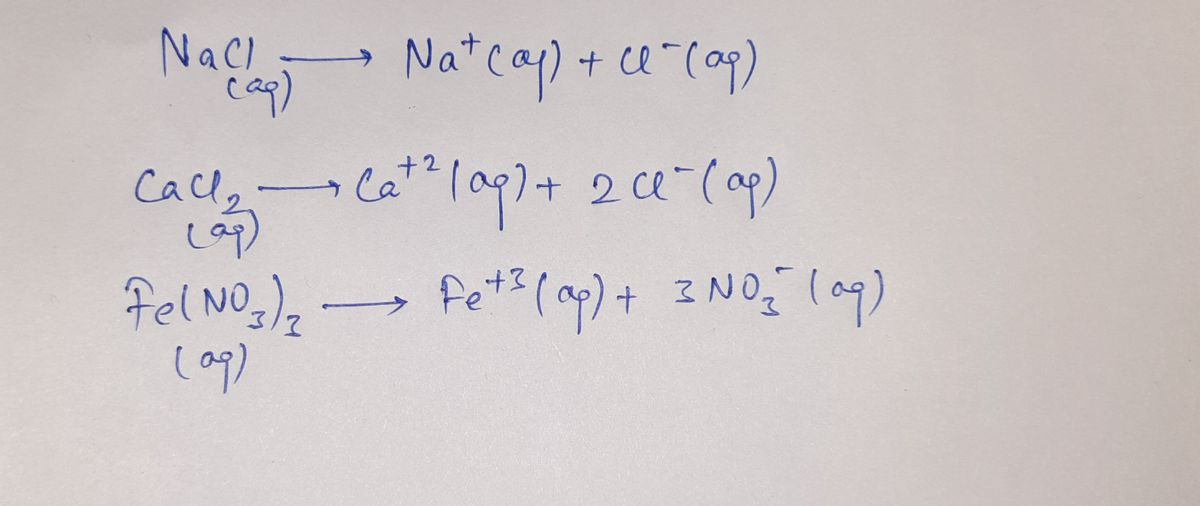
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY