Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter16: Spontaneity Of Reaction
Section: Chapter Questions
Problem 69QAP: At 25C, a 0.327 M solution of a weak acid HX has a pH of 5.12. What is G for the dissociation of the...
Related questions
Question
How can we solve these two questions?

Transcribed Image Text:Macmillan Learning
If the K₂ of a monoprotic weak acid is 3.3 x 10-6, what is the pH of a 0.41 M solution of this acid?
pH =
x10
TOOLS
O Search
![Learning
A monoprotic weak acid, HA, dissociates in water according to the reaction
HA(aq) + H₂O(1) H₂O+ (aq) + A (aq)
The equilibrium concentrations of the reactants and products are [HA] = 0.160 M, [H3O+] = 2.00 × 10-4 M, and
[A-]=2.00 x 10-4 M. Calculate the K, value for the acid HA.
K₁ =
H
O Search](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F9437567b-8da5-455d-a4fe-2bd0f454b63f%2Ff9622b55-7336-419c-8f43-fee169bebfae%2Fgrmce4o_processed.jpeg&w=3840&q=75)
Transcribed Image Text:Learning
A monoprotic weak acid, HA, dissociates in water according to the reaction
HA(aq) + H₂O(1) H₂O+ (aq) + A (aq)
The equilibrium concentrations of the reactants and products are [HA] = 0.160 M, [H3O+] = 2.00 × 10-4 M, and
[A-]=2.00 x 10-4 M. Calculate the K, value for the acid HA.
K₁ =
H
O Search
Expert Solution
Step 1
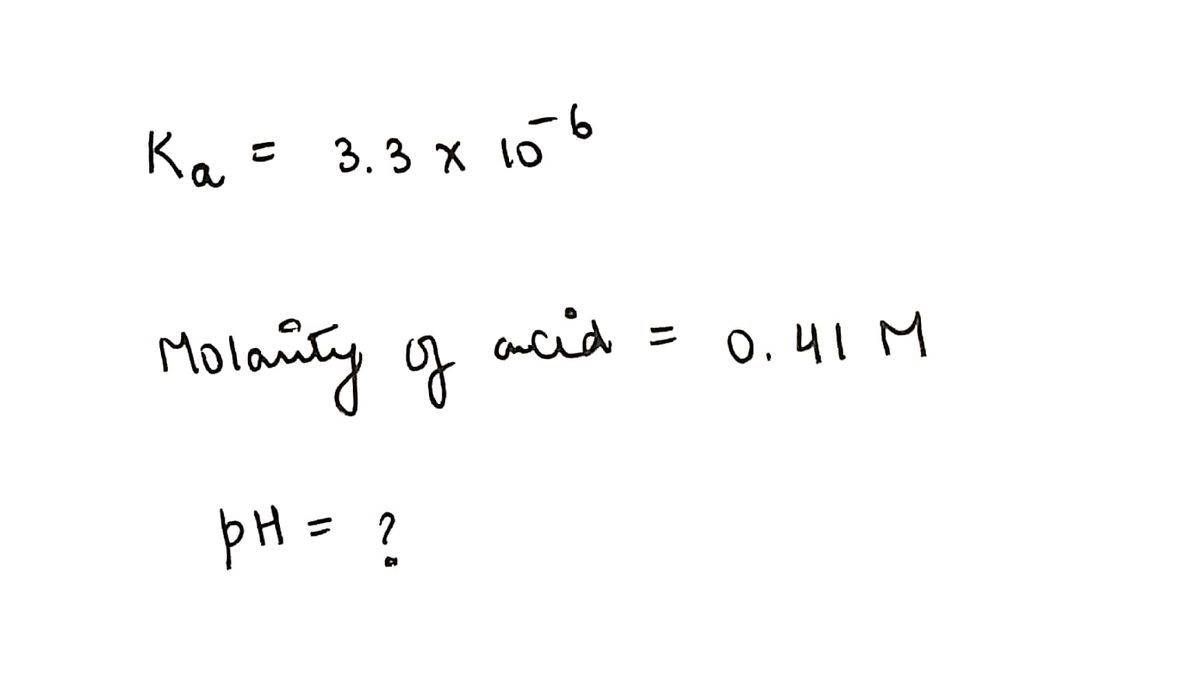
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning