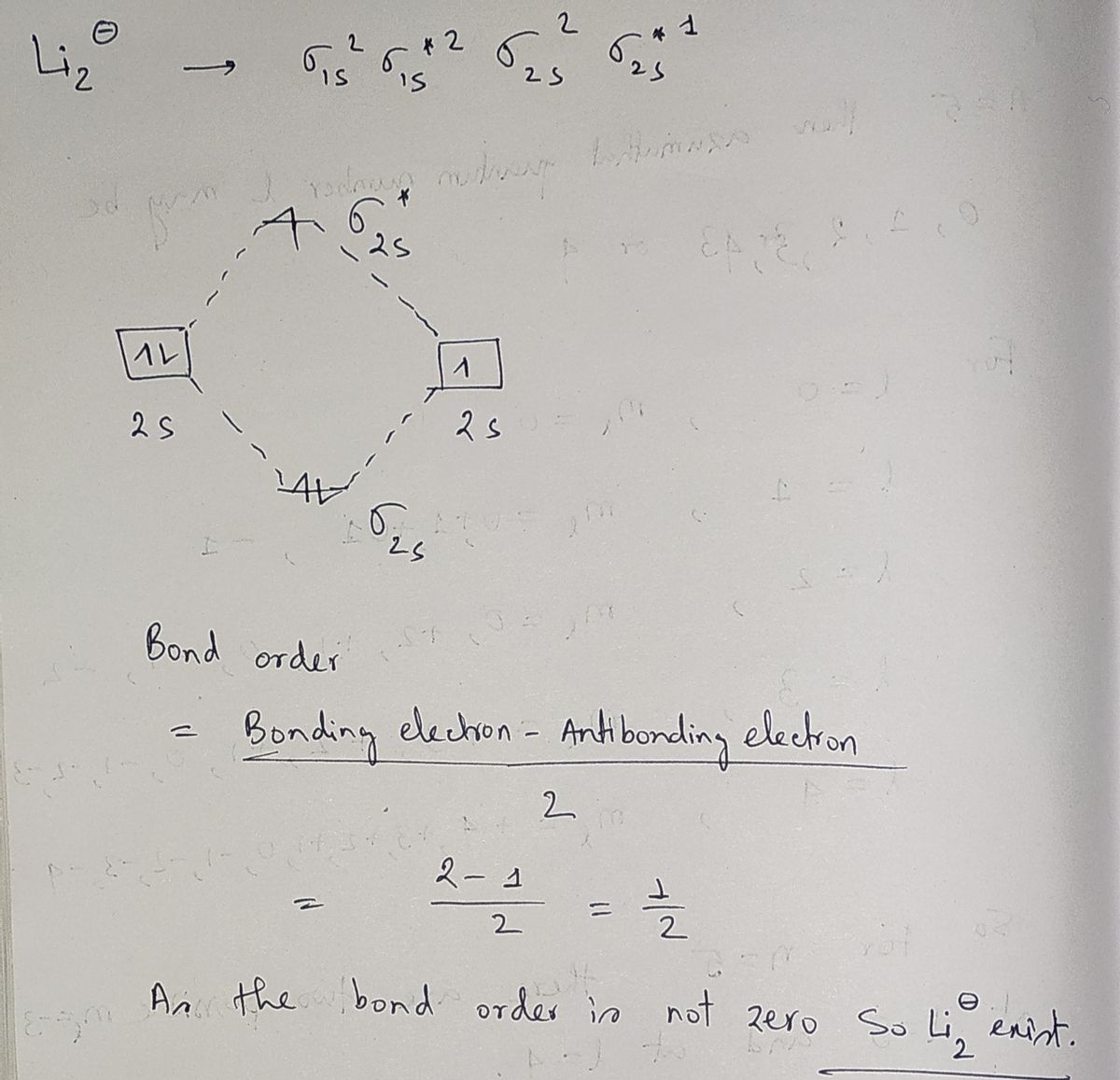
Label the valence AOs shown, build from them a molecular orbital diagram for each diatomic particle, label MOs by their type, fill in the valence e s as arrows, calculate the MO bond order, and decide if the diatomic may exist or not: MO diagram for [LizL MO diagram for [Liz]* AO of Li MOs of [Liz AO of Li AO of Li MOs of [Liz]* AO of Lit MO bond order: Exists? MO bond order: Exists?
Label the valence AOs shown, build from them a molecular orbital diagram for each diatomic particle, label MOs by their type, fill in the valence e s as arrows, calculate the MO bond order, and decide if the diatomic may exist or not: MO diagram for [LizL MO diagram for [Liz]* AO of Li MOs of [Liz AO of Li AO of Li MOs of [Liz]* AO of Lit MO bond order: Exists? MO bond order: Exists?
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
![**Instructions for Constructing Molecular Orbital Diagrams**
The task involves creating molecular orbital (MO) diagrams for diatomic molecules, specifically \([ \text{Li}_2 ]^-\) and \([ \text{Li}_2 ]^+\). Follow these steps:
1. **Labeling Valence Atomic Orbitals (AOs)**:
- Identify and label valence atomic orbitals for lithium (\(\text{Li}\)) which generally includes the 2s orbital.
2. **Building Molecular Orbital Diagrams**:
- Combine the valence AOs of two lithium atoms to form MOs, labeling them accordingly (e.g., \(\sigma\) or \(\sigma^*\)).
3. **Filling in Valence Electrons**:
- For each molecule, fill in the electrons in the MOs using arrows to represent electrons. Consider the charge on the molecule for the correct electron count:
- \([ \text{Li}_2 ]^-\) will have an extra electron.
- \([ \text{Li}_2 ]^+\) will have one less electron.
4. **Calculating MO Bond Order**:
- Use the formula: \[
\text{Bond Order} = \frac{\text{Number of bonding electrons} - \text{Number of antibonding electrons}}{2}
\]
5. **Determining Existence**:
- A positive bond order suggests the diatomic molecule may exist, while a bond order of zero or negative indicates it likely does not.
**Components in the Diagrams**:
- **AO of Li**: The atomic orbitals of a single lithium atom, typically the 2s orbital.
- **MOs of \([ \text{Li}_2 ]^-\)** and \([ \text{Li}_2 ]^+\)**: The molecular orbitals produced by combining AOs of two lithium atoms.
Each diagram should be completed based on the defined steps, and the MO bond order should be calculated to assess potential existence.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F7e6fe4d9-c522-461f-bc62-94d23fd62501%2F5b8f9add-2787-4cba-a574-bd130ca07aaa%2F4bu7o54_processed.png&w=3840&q=75)
Transcribed Image Text:**Instructions for Constructing Molecular Orbital Diagrams**
The task involves creating molecular orbital (MO) diagrams for diatomic molecules, specifically \([ \text{Li}_2 ]^-\) and \([ \text{Li}_2 ]^+\). Follow these steps:
1. **Labeling Valence Atomic Orbitals (AOs)**:
- Identify and label valence atomic orbitals for lithium (\(\text{Li}\)) which generally includes the 2s orbital.
2. **Building Molecular Orbital Diagrams**:
- Combine the valence AOs of two lithium atoms to form MOs, labeling them accordingly (e.g., \(\sigma\) or \(\sigma^*\)).
3. **Filling in Valence Electrons**:
- For each molecule, fill in the electrons in the MOs using arrows to represent electrons. Consider the charge on the molecule for the correct electron count:
- \([ \text{Li}_2 ]^-\) will have an extra electron.
- \([ \text{Li}_2 ]^+\) will have one less electron.
4. **Calculating MO Bond Order**:
- Use the formula: \[
\text{Bond Order} = \frac{\text{Number of bonding electrons} - \text{Number of antibonding electrons}}{2}
\]
5. **Determining Existence**:
- A positive bond order suggests the diatomic molecule may exist, while a bond order of zero or negative indicates it likely does not.
**Components in the Diagrams**:
- **AO of Li**: The atomic orbitals of a single lithium atom, typically the 2s orbital.
- **MOs of \([ \text{Li}_2 ]^-\)** and \([ \text{Li}_2 ]^+\)**: The molecular orbitals produced by combining AOs of two lithium atoms.
Each diagram should be completed based on the defined steps, and the MO bond order should be calculated to assess potential existence.
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY