Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
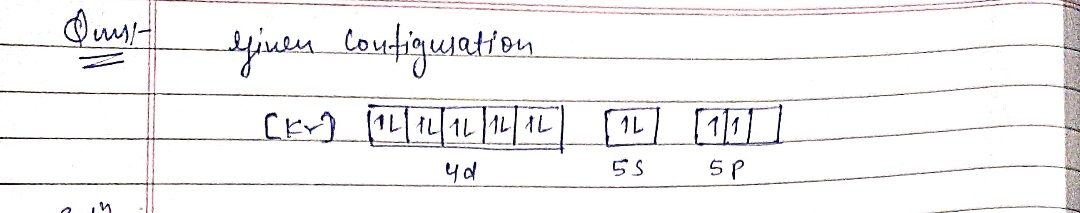
What element is represented? Is it dimagnetic or paramagnetic? What is a possible set of quantum numbers for an unpaired electron in the orbital box diagram?
![The image depicts an electron configuration using an orbital diagram format. It begins with the electron configuration of krypton, denoted by [Kr], and continues with additional electrons placed in higher energy orbitals.
- **4d Orbital**: Contains five boxes, each representing an orbital. The electrons are distributed as follows:
- First orbital: Two electrons with opposite spins.
- Second orbital: Two electrons with opposite spins.
- Third orbital: Two electrons with opposite spins.
- Fourth orbital: One electron with an upward spin.
- Fifth orbital: One electron with an upward spin.
- **5s Orbital**: Contains one box.
- Two electrons with opposite spins.
- **5p Orbital**: Contains three boxes.
- First box: One electron with an upward spin.
- Second and third boxes are empty.
This diagram represents the filling of electron orbitals following Hund's Rule and the Pauli Exclusion Principle.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F875f71cc-9adf-413c-aafc-a07abb8e0bf0%2Fb219d3b4-6bb3-42c2-8bd1-d9593d89df62%2Feluj4i5_processed.png&w=3840&q=75)
Transcribed Image Text:The image depicts an electron configuration using an orbital diagram format. It begins with the electron configuration of krypton, denoted by [Kr], and continues with additional electrons placed in higher energy orbitals.
- **4d Orbital**: Contains five boxes, each representing an orbital. The electrons are distributed as follows:
- First orbital: Two electrons with opposite spins.
- Second orbital: Two electrons with opposite spins.
- Third orbital: Two electrons with opposite spins.
- Fourth orbital: One electron with an upward spin.
- Fifth orbital: One electron with an upward spin.
- **5s Orbital**: Contains one box.
- Two electrons with opposite spins.
- **5p Orbital**: Contains three boxes.
- First box: One electron with an upward spin.
- Second and third boxes are empty.
This diagram represents the filling of electron orbitals following Hund's Rule and the Pauli Exclusion Principle.
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY