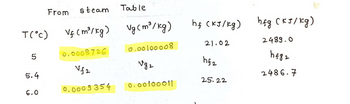Saturated water at 36.6°C passes through a throttling valve and becomes a mixture of water and steam at 5.4°C. Calculate the specific volume and specific enthalpy of the mixture. Show detailed steps of how the properties are obtained by using only the Saturated Water and Steam table. Consider that a throttling process is a typical irreversible process. The specific enthalpies of the working fluid before and after a throttling valve are the same.
Saturated water at 36.6°C passes through a throttling valve and becomes a mixture of water and steam at 5.4°C. Calculate the specific volume and specific enthalpy of the mixture. Show detailed steps of how the properties are obtained by using only the Saturated Water and Steam table.
Consider that a throttling process is a typical irreversible process. The specific enthalpies of the working fluid before and after a throttling valve are the same.
The steam table has been provided in the image

Step by step
Solved in 3 steps with 3 images

I have some things that need clarification based on the answer provided to this question. Firstly, the h1 value came out as 154.99 kJ/Kg for me not 153.318. Also, the values taken from the steam table for Vf and Vg for 5 degrees celcius and 6 degrees are different from what is on the steam table, I have attached a picture for this. For 5 degrees on the steam table it shows Vf as 0.00100008 and Vg as 147.011 and for 6 degrees it shows Vf as 0.00100011 and Vg as 137.633. Please clarify this and reply as soon as possible. Thanks