Is this methoxybenzoic or methylbenzoic? Someone else said it was methoxybenzoic because the single peak wasn't present for a methyl group but looks like to me that it is. Are there other differences? Including the IR and HNMR spectrums.
Is this methoxybenzoic or methylbenzoic? Someone else said it was methoxybenzoic because the single peak wasn't present for a methyl group but looks like to me that it is. Are there other differences? Including the IR and HNMR spectrums.


First, let's understand all the peaks that can be obtained from both compounds.
Methylbenzoic acid:
Here, para-methyl benzoic acid is considered for demonstration purposes.
All the chemically different protons in this compound are shown below:
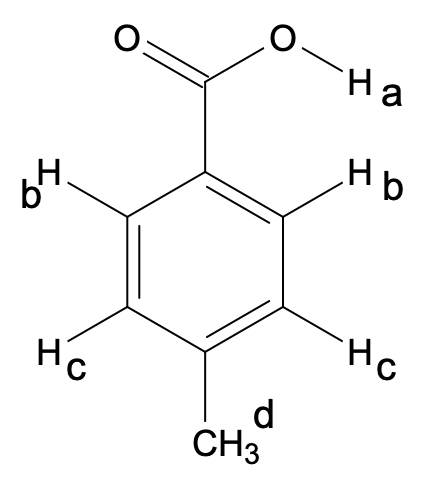
Thus, there are 4 types of different protons (a, b, c, d) which will lead to 4 1H-NMR signals.
Methoxybenzoic acid:
Again, the para compound has been taken into consideration for demonstration purposes.
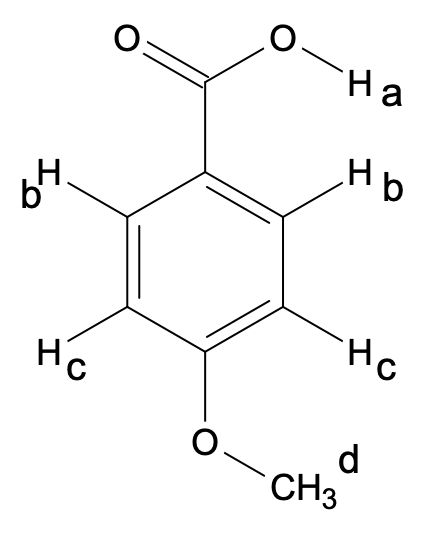
Here also, 4 types of chemically non-equivalent protons are there that will give rise to 4 NMR signals.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images









