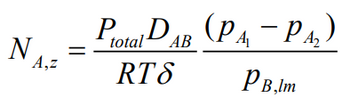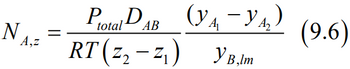In a rectification column, toluene is diffusing from gas to liquid and benzene from liquid to gas at temperature of 30°C and pressure 101.3 kN/m2 under conditions of equal molar counter diffusion. At one point in the column, the molal concentrations of toluene on the two sides of a gas film thick 0.5 mm are 80% and 20%, respectively. Assuming the diffusivity of benzenetoluene vapour under the operating conditions to be 0.38 x 10-5 m2/s, estimate the rate of diffusion of toluene and benzene in kg/hr across an area of 0.01 m2. The molecular weight of toluene and benzene are 92 and 78 g/mol, respectively. The ideal gas constant is equal to 8.314 m3⋅Pa⋅K−1⋅mol−1
In a rectification column, toluene is diffusing from gas to liquid and benzene from liquid to gas at temperature of 30°C and pressure 101.3 kN/m2 under conditions of equal molar counter
diffusion. At one point in the column, the molal concentrations of toluene on the two sides of
a gas film thick 0.5 mm are 80% and 20%, respectively. Assuming the diffusivity of benzenetoluene vapour under the operating conditions to be 0.38 x 10-5 m2/s, estimate the rate of diffusion of toluene and benzene in kg/hr across an area of 0.01 m2. The molecular weight of toluene and benzene are 92 and 78 g/mol, respectively. The ideal gas constant is equal to 8.314 m3⋅Pa⋅K−1⋅mol−1
.
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Couldn't you use these equations