If a throttling valve is connected to the outlet for one of these tanks, whereby air leaves this valve steadily at 305K with a pressure drop of 50bar, apply an exergy balance across the valve and calculate the exergy change (units: kJ/kg)
If a throttling valve is connected to the outlet for one of these tanks, whereby air leaves this valve steadily at 305K with a pressure drop of 50bar, apply an exergy balance across the valve and calculate the exergy change (units: kJ/kg)
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
If a throttling valve is connected to the outlet for one of these tanks, whereby air leaves this valve steadily at 305K with a pressure drop of 50bar, apply an exergy balance across the valve and calculate the exergy change (units: kJ/kg).

Transcribed Image Text:CAES-Background Info to (Calculated) Questions
A small-scale Compressed Air Energy Storage (CAES) system uses gas tank storage,
with any single tank sized to carry a maximum 2000kg air. The air storage helps
support various fluid power (pneumatics) requirements, but also serves for energy
storage (at 100% charge).
The CAES system sits in a plant room at a geographical location with an environmental
temperature of 300K and ambient pressure of 1 atmosphere (101.325kPa). Due to the
presence of other equipment in the plant room and its built-up nature, is the
immediate surroundings from which tanks are filled are normally a little warmer
compared to the outside ambient air (but same ambient pressure). The plant room
itself is at 310K.The specification for a single tank stipulates it can only be filled with
(dry) air to a maximum (gauge) pressure of 500bar (at typical operating conditions).
When left inside the plant room, the CAES system eventually attains the plant room
temperature.
You may assume:
Steady state conditions apply
Negligible changes in kinetic and potential energy
• Thermal mass of the tank (metal) is negligible
• Air behaves as an ideal gas
●
A gas tank is rigid
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
the pressure dropped by 50 bar and the initial was 500 bar so P2 should be 450 bar. Also, how did u get this ln50500
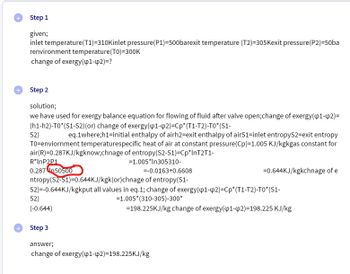
Transcribed Image Text:Step 1
given;
inlet temperature(T1)=310Kinlet pressure(P1)=500barexit temperature (T2)=305Kexit pressure(P2)=50ba
renvironment temperature(TO)=300K
change of exergy(41-42)=?
Step 2
solution;
we have used for exergy balance equation for flowing of fluid after valve open;change of exergy(41-42)=
(h1-h2)-TO*(S1-S2)(or) change of exergy(41-42)=Cp*(T1-T2)-T0*(S1-
eq.1where;h1=initial enthalpy of airh2=exit enthalpy of airS1=inlet entropyS2-exit entropy
TO=enviornment temperaturespecific heat of air at constant pressure(Cp)=1.005 KJ/kgkgas constant for
air(R)=0.287KJ/kgknow;chnage of entropy(S2-S1)=Cp*lnT2T1-
S2)
R*InP2P1
0.287 50500
S2)
(-0.644)
=1.005*In305310-
=-0.0163+0.6608
ntropy(S2-S1)=0.644KJ/kgk(or)chnage of entropy(S1-
S2)=-0.644KJ/kgkput all values in eq.1; change of exergy(41-q2)=Cp* (T1-T2)-T0*(S1-
=1.005* (310-305)-300*
=198.225KJ/kg change of exergy(p1-q2)=198.225 KJ/kg
Step 3
=0.644KJ/kgkchnage of e
answer;
change of exergy(p1-p2)=198.225KJ/kg
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY