Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
100%
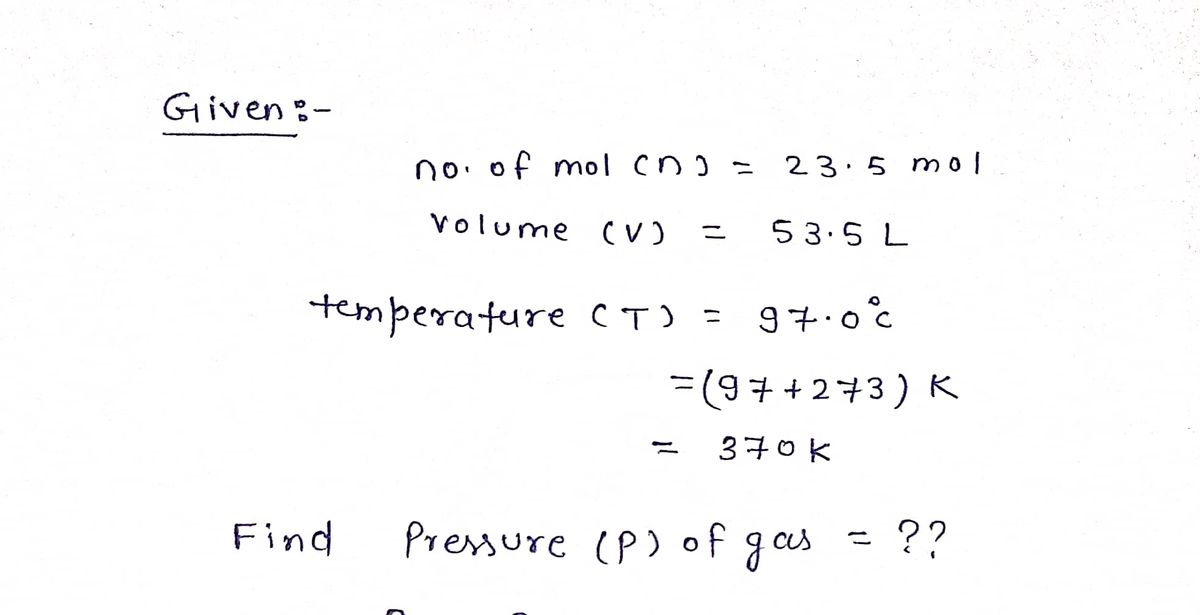
![**Problem Statement:**
If 23.5 mol of an ideal gas occupies 53.5 L at 97.00 °C, what is the pressure of the gas?
**Solution:**
To find the pressure, \( P \), use the Ideal Gas Law:
\[ PV = nRT \]
Where:
- \( P \) is the pressure in atmospheres (atm),
- \( V \) is the volume in liters (L),
- \( n \) is the number of moles (mol),
- \( R \) is the ideal gas constant, \( 0.0821 \, \text{L atm/mol K} \),
- \( T \) is the temperature in Kelvin (K).
**Calculation Steps:**
1. Convert the temperature from Celsius to Kelvin:
\[ T(K) = 97.00 + 273.15 = 370.15 \, \text{K} \]
2. Substitute the values into the Ideal Gas Law and solve for \( P \):
\[ P = \frac{nRT}{V} = \frac{(23.5 \, \text{mol})(0.0821 \, \text{L atm/mol K})(370.15 \, \text{K})}{53.5 \, \text{L}} \]
3. Calculate \( P \) using the values provided.
**Final Answer:**
\[ P = \boxed{ } \, \text{atm} \]](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F6797ee8f-35b0-45f9-be91-6b4961db7d8a%2F0dfb9b4d-b655-489d-ad3a-ca45aeb5252c%2Fgqk7eb_processed.png&w=3840&q=75)
Transcribed Image Text:**Problem Statement:**
If 23.5 mol of an ideal gas occupies 53.5 L at 97.00 °C, what is the pressure of the gas?
**Solution:**
To find the pressure, \( P \), use the Ideal Gas Law:
\[ PV = nRT \]
Where:
- \( P \) is the pressure in atmospheres (atm),
- \( V \) is the volume in liters (L),
- \( n \) is the number of moles (mol),
- \( R \) is the ideal gas constant, \( 0.0821 \, \text{L atm/mol K} \),
- \( T \) is the temperature in Kelvin (K).
**Calculation Steps:**
1. Convert the temperature from Celsius to Kelvin:
\[ T(K) = 97.00 + 273.15 = 370.15 \, \text{K} \]
2. Substitute the values into the Ideal Gas Law and solve for \( P \):
\[ P = \frac{nRT}{V} = \frac{(23.5 \, \text{mol})(0.0821 \, \text{L atm/mol K})(370.15 \, \text{K})}{53.5 \, \text{L}} \]
3. Calculate \( P \) using the values provided.
**Final Answer:**
\[ P = \boxed{ } \, \text{atm} \]
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY