Identify the cyclohexyl substrate which would undergo the slowest E2 with base.

To identify the cyclohexyl substrate which would undergo the slowest E2 with base.
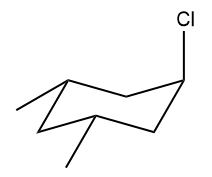
For E2 elimination reaction of cyclohexyl substrate it is required that the leaving group must be axial. If the leaving group is at equatorial then flip the chair so that the leaving group is axial.
Also, if the substitute group is axial then it makes the cyclohexyl conformation less stable and if the substitute group is equatorial then it makes the cyclohexyl conformation more stable.
The rate of E2 reaction is slowest if the cyclohexyl substrate (in which leaving group is axial) is unstable and the rate of E2 reaction is fastest if the cyclohexyl substrate is stable.
Thus, the slowest E2 reaction is given by those cyclohexyl substrate in which the conformation having Cl at axial have higher number of axial substituents.
So, identify stability by counting the axial substituent in the conformation where the Cl is axial. If it is not axial then flip the ring.
In (A) there is zero axial substitute therefore it is stable conformation. Hence reaction rate is fast.
Step by step
Solved in 8 steps with 5 images









