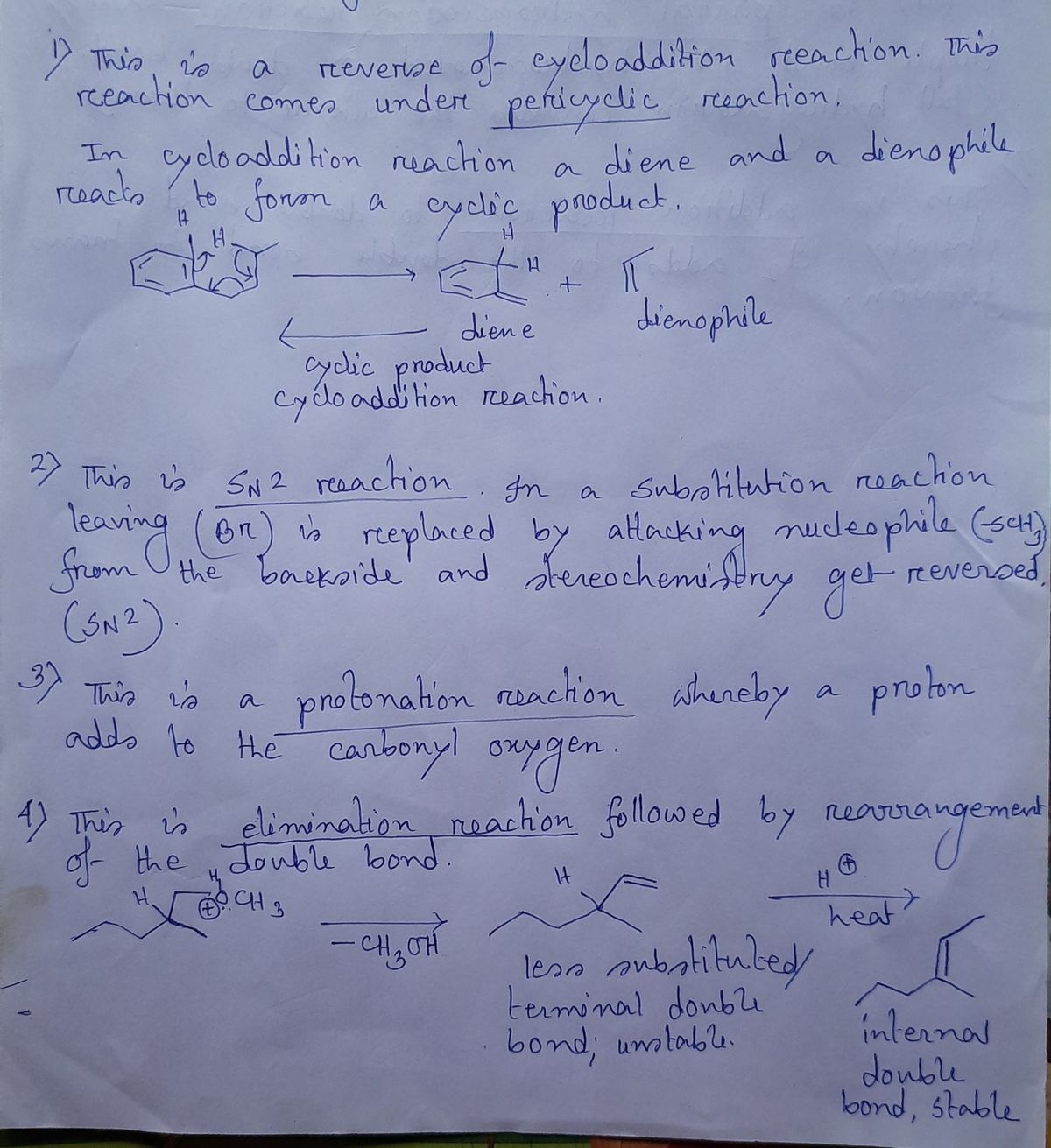
Identify each of the following reactions types SCH3 Br H J. F. + + محمد NaSCH; OH Ethanol NHẠCH H -OCH3 © CH3 H₂C-CH₂ 0₂₁₂ H₂SO4 heat H3C of You - OH + NH₂ to CH3 OH Acetic acid (Vinegar is 5% acetic acid in water) NaBr
Identify each of the following reactions types SCH3 Br H J. F. + + محمد NaSCH; OH Ethanol NHẠCH H -OCH3 © CH3 H₂C-CH₂ 0₂₁₂ H₂SO4 heat H3C of You - OH + NH₂ to CH3 OH Acetic acid (Vinegar is 5% acetic acid in water) NaBr
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter20: Organic Chemistry
Section: Chapter Questions
Problem 21A
Related questions
Question

Transcribed Image Text:**Identify Each of the Following Reaction Types**
1. **First Reaction:**
- **Type:** Dehydrohalogenation
- **Reactants:** Cyclohexyl bromide and a base
- **Products:** Cyclohexene and HBr
- **Description:** The hydrogen atom and the bromine atom are removed from the adjacent carbon atoms, resulting in the formation of a double bond and cyclohexene.
2. **Second Reaction:**
- **Type:** Nucleophilic Substitution (SN2)
- **Reactants:** Cyclohexyl bromide and sodium thiomethoxide (NaSCH3)
- **Products:** Cyclohexyl methyl sulfide and sodium bromide
- **Description:** The bromine atom is replaced by a methoxysulfide group (-SCH3).
3. **Third Reaction:**
- **Type:** Ammonolysis
- **Reactants:** Butanone and ammonium chloride (NH4Cl)
- **Products:** Butylammonium chloride and ammonia (NH3)
- **Description:** The ketone reacts with ammonia forming a primary amine.
4. **Fourth Reaction:**
- **Type:** Dehydration
- **Reactants:** Alcohol and sulfuric acid (H2SO4) with heat
- **Products:** Alkene
- **Description:** Removal of water (H2O) from 3-methyl-2-pentanol produces an alkene (3-methyl-1-pentene).
5. **Fifth Reaction:**
- **Type:** Oxidation
- **Reactants:** Ethanol and oxygen (O2)
- **Products:** Acetic acid
- **Description:** Ethanol is oxidized to acetic acid, which is a major component of vinegar.
6. **Sixth Reaction:**
- **Type:** Hydrogenation
- **Reactants:** Alkene and hydrogen (H2) with Lindlar catalyst
- **Products:** Alkane
- **Description:** The triple bond is partially reduced to a double bond due to the Lindlar catalyst, resulting in partial hydrogenation of alkyne to alkene.
7. **Seventh Reaction:**
- **Type:** Halogenation
- **Reactants:** Cyclopentene and chlorine (Cl2
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning