Ice cubes at 0 C are poured into a glass that initially contains 150 g of liquid water at 20 C. The contents of the glass are gently stirred stirred and after a short time some of the ice melts and the liquid cools to 0 C. No apppreciable heat exchange with the environment takes place during this process. (a) Calculate the entropy change of the universe during the process. Use only the following data (no external data sources): The heat capacity of liquid water is 4.18 J/g*K, which you may assume to be independent of temperature over the range of interest. For liquids, Cp=Cv) (b) If the same process were performed reversibly, how much useful work would be obtained from it. Any required heat exchange to achieve the same final state reversibly should be with the environment at 20 C.
Ice cubes at 0 C are poured into a glass that initially contains 150 g of liquid water at 20 C. The contents of the glass are gently stirred stirred and after a short time some of the ice melts and the liquid cools to 0 C. No apppreciable heat exchange with the environment takes place during this process.
(a) Calculate the entropy change of the universe during the process. Use only the following data (no external data sources): The heat capacity of liquid water is 4.18 J/g*K, which you may assume to be independent of temperature over the range of interest. For liquids, Cp=Cv)
(b) If the same process were performed reversibly, how much useful work would be obtained from it. Any required heat exchange to achieve the same final state reversibly should be with the environment at 20 C.
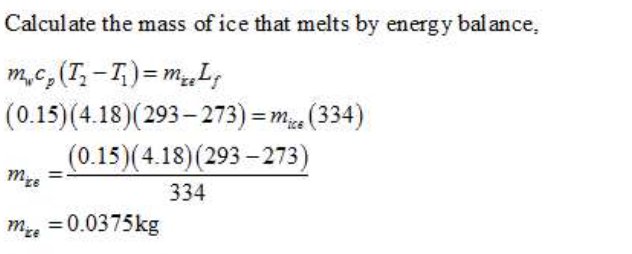
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images









