I need help with 2 but 1 tells you other measurements
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question
100%
I need help with 2 but 1 tells you other measurements
![**Procedure:**
1. **Preparation of CuSO₄ Solution:**
- Begin by measuring approximately 0.40 g of copper (II) sulfate (CuSO₄) to make a solution. Note that the formula refers to anhydrous CuSO₄, but if instead, a blue crystalline solid is provided, it indicates CuSO₄·5H₂O. If you're unsure, ask your instructor since the molecular weight of the solid will differ.
- Dissolve the measured CuSO₄ in 300-400 mL of water. If the solution appears light blue-green, it is likely CuSO₄·5H₂O.
2. **Calculation of NaOH Solution Volume:**
- Use the mass of your solid to calculate the volume of 0.20 M NaOH solution required to fully react with the CuSO₄. Adjust the calculation to ensure the copper is the limiting reactant, avoiding excess NaOH.
- The formula used is:
\[
\text{Volume of NaOH} = \frac{\text{mass of CuSO₄} \times \text{molarity of CuSO₄}}{\text{molarity of NaOH}}
\]
- This formula ensures you add an appropriate amount of NaOH solution.
3. **Addition of NaOH Solution:**
- Carefully add the calculated volume of NaOH solution to the beaker containing the dissolved CuSO₄. Stir gently, then allow the solid to settle.
4. **Inspection and Adjustment:**
- After the precipitate has settled, inspect the solution. If it is still tinted blue, it indicates some copper ions did not react. In such a case, allow it to settle further and add a few more mL of NaOH until the solution becomes clear, indicating all copper has been precipitated. If it becomes clear before adding excess NaOH, this means the reaction is complete.
_Note: It is fine to slightly overdo the addition of NaOH to ensure complete reaction._](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F6720f477-d171-4017-aeea-4a98f198692e%2F27bd8b13-1fc6-4e19-aaff-e824a1d64ed6%2F8u5099k.jpeg&w=3840&q=75)
Transcribed Image Text:**Procedure:**
1. **Preparation of CuSO₄ Solution:**
- Begin by measuring approximately 0.40 g of copper (II) sulfate (CuSO₄) to make a solution. Note that the formula refers to anhydrous CuSO₄, but if instead, a blue crystalline solid is provided, it indicates CuSO₄·5H₂O. If you're unsure, ask your instructor since the molecular weight of the solid will differ.
- Dissolve the measured CuSO₄ in 300-400 mL of water. If the solution appears light blue-green, it is likely CuSO₄·5H₂O.
2. **Calculation of NaOH Solution Volume:**
- Use the mass of your solid to calculate the volume of 0.20 M NaOH solution required to fully react with the CuSO₄. Adjust the calculation to ensure the copper is the limiting reactant, avoiding excess NaOH.
- The formula used is:
\[
\text{Volume of NaOH} = \frac{\text{mass of CuSO₄} \times \text{molarity of CuSO₄}}{\text{molarity of NaOH}}
\]
- This formula ensures you add an appropriate amount of NaOH solution.
3. **Addition of NaOH Solution:**
- Carefully add the calculated volume of NaOH solution to the beaker containing the dissolved CuSO₄. Stir gently, then allow the solid to settle.
4. **Inspection and Adjustment:**
- After the precipitate has settled, inspect the solution. If it is still tinted blue, it indicates some copper ions did not react. In such a case, allow it to settle further and add a few more mL of NaOH until the solution becomes clear, indicating all copper has been precipitated. If it becomes clear before adding excess NaOH, this means the reaction is complete.
_Note: It is fine to slightly overdo the addition of NaOH to ensure complete reaction._
Expert Solution
Step 1 Introduction
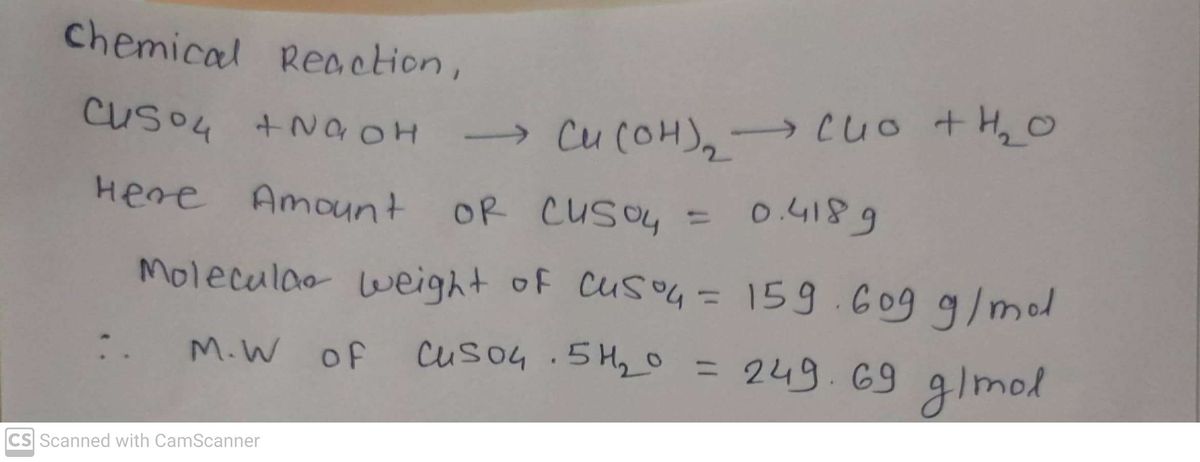
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON