I need help step step on how tpo calculate (In). I have no idea how to get this number. The decomposition of hydrogen peroxide in dilute sodium hydroxide at 20 °C H2O2(aq) H2O(l) + ½ O2(g) is first order in H2O2 with a rate constant of .00110 min-1. If the initial concentration of H2O2 is .0302M, the concentration of H2O2 will be __________M after 1144.0 min have passed.
I need help step step on how tpo calculate (In). I have no idea how to get this number.
The decomposition of hydrogen peroxide in dilute sodium hydroxide at 20 °C
H2O2(aq) H2O(l) + ½ O2(g)
is first order in H2O2 with a rate constant of .00110 min-1.
If the initial concentration of H2O2 is .0302M, the concentration of H2O2 will be __________M after 1144.0 min have passed.
Given, the decomposition of hydrogen peroxide in dilute sodium hydroxide at 20 °C follows a first order kinetics.
We know that, rate constant for a first order reaction can be expressed as,
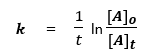
where, k is the rate constant
t is time
[A]o is the initial concentration = 5 ppm
[A]t is the concentration at time t.
Step by step
Solved in 2 steps with 2 images









