3. Water is flowing at an average velocity of 7 ft/s in a 6-in pipe. What is the pressure drop per nit length
3. Water is flowing at an average velocity of 7 ft/s in a 6-in pipe. What is the pressure drop per nit length
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
3

Transcribed Image Text:|
3. Water is flowing at an average velocity of 7 ft/s in a 6-in pipe. What is the pressure drop per
unit length
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
i am confused on where you are getting these equations.
is f--- friction? why is it 64/row VD/viscosity?
are there other units at play here?
i am not seeing how they cancel out to become Pa/m
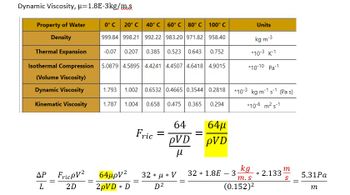
Transcribed Image Text:Dynamic Viscosity, μ-1.8E-3kg/m.s
Property of Water
Density
0°C 20° C
40° C 60° C 80° C 100° C
999.84 998.21
992.22
983.20 971.82
958.40
Thermal Expansion
-0.07 0.207 0.385
0.523 0.643 0.752
Isothermal Compression 5.0879 4.5895 4.4241
4.4507 4.6418 4.9015
(Volume Viscosity)
Dynamic Viscosity
Kinematic Viscosity
ΔΡ
L
=
FricpV²
2D
1.793 1.002 0.6532 0.4665 0.3544 0.2818 10-3 kg m-1 s-1 (Pa s)
1.787 1.004 0.658 0.475 0.365 0.294
*10-6 m² s-1
64μpV²
2pVD * D
Fric =
64
OVD
μ
32 *μ* V
D²
=
64μ
pVD
Units
kg m-3
*10-3 K-1
*10-10 Pa-1
kg
m. s
(0.152)²
32 1.8E 3
-
* 2.133
m
S
5.31Pa
m
Solution
Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The