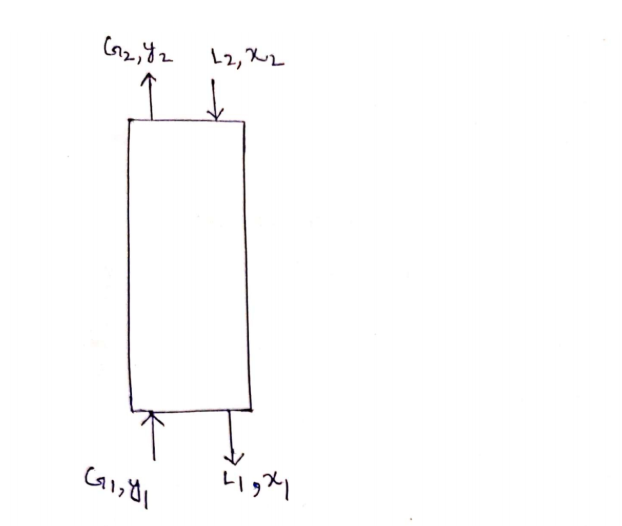
Hydrogen sulfide (H2S) gas in petroleum refinery is to be removed from a 1387kg/h gas mixture consist of 16 % w.w H2S into air, using counter current absorption column. Water is used as solvent in the absorption column. The water flow rate is 3 times the gas mixture flow rate. The air mole percent at the top of the column is 97.5%. The air consists of 21.9% oxygen and 78.1% nitrogen. Calculate; 4. The mole fraction of H2S in the liquid stream leaving the bottom of the tower? Note; Don’t round numbers; Minimum 4 decimals (0.0000) Given A) The air consists of 21.9% oxygen and 78.1% nitrogen. B) The atomic weight of; 1. The hydrogen atom is 1 2. The Oxygen atom is 16 3. The sulfur atom is 32 4. 1The nitrogen atom is 14
Hydrogen sulfide (H2S) gas in petroleum refinery is to be removed from a 1387kg/h gas mixture consist of 16 % w.w H2S into air, using counter current absorption column.
Water is used as solvent in the absorption column. The water flow rate is 3 times the gas mixture flow rate.
The air mole percent at the top of the column is 97.5%.
The air consists of 21.9% oxygen and 78.1% nitrogen.
Calculate;
4. The mole fraction of H2S in the liquid stream leaving the bottom of the tower?
Note; Don’t round numbers; Minimum 4 decimals (0.0000)
Given
A) The air consists of 21.9% oxygen and 78.1% nitrogen.
B) The atomic weight of;
1. The hydrogen atom is 1
2. The Oxygen atom is 16
3. The sulfur atom is 32
4. 1The nitrogen atom is 14

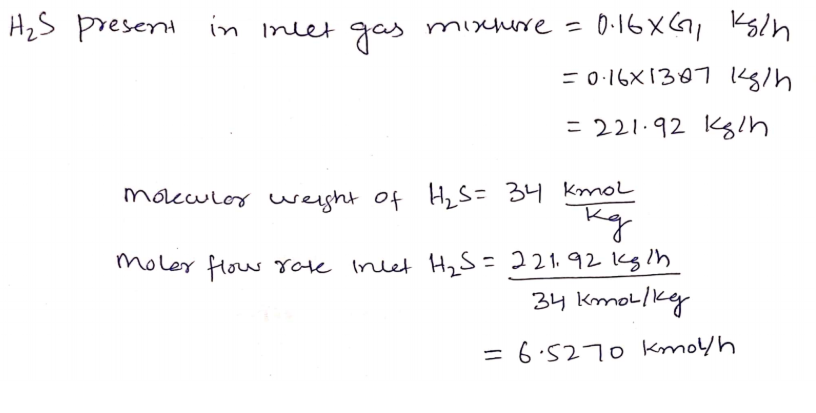
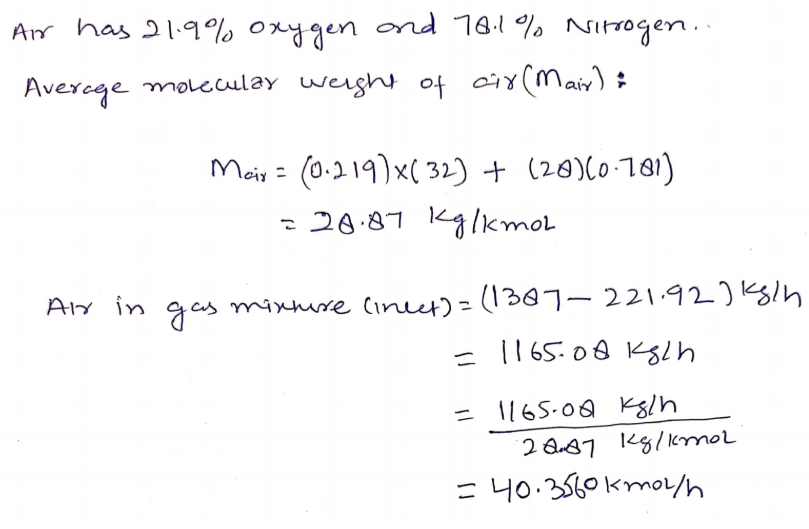
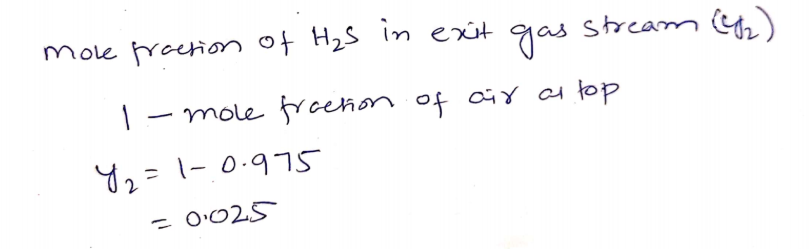

Step by step
Solved in 8 steps with 10 images









