Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
100%

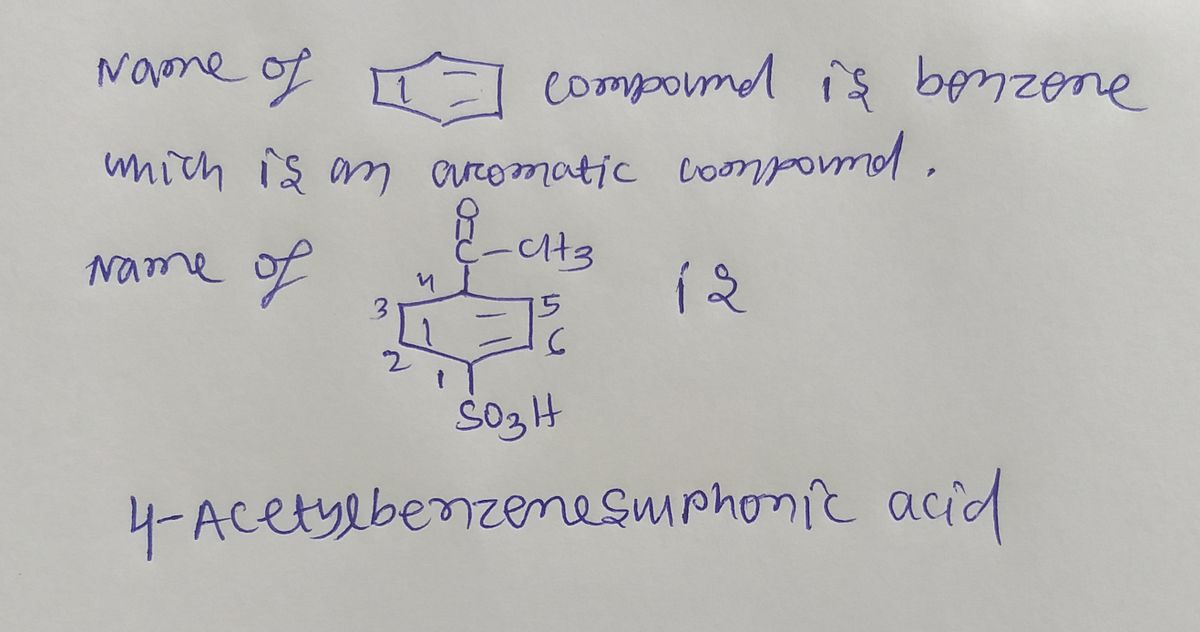
Transcribed Image Text:**Title: Synthesis of Acetylbenzenesulfonic Acid from Benzene**
**Objective:**
Discuss the process of synthesizing acetylbenzenesulfonic acid from benzene.
**Chemical Structures:**
1. **Reactant:**
- **Benzene:** Represented by a simple hexagonal ring structure indicating six carbon atoms linked in a planar ring, with alternating single and double bonds symbolizing aromaticity.
2. **Product:**
- **Acetylbenzenesulfonic Acid:**
- Aromatic ring similar to benzene.
- Substituents:
- An acyl group (C=OCH₃) attached to the top of the benzene ring, representing acetylation.
- A sulfonic acid group (SO₃H) attached to the bottom.
**Synthesis Process Overview:**
To achieve the transformation from benzene to acetylbenzenesulfonic acid, the following steps could be involved:
1. **Sulfonation:**
- *Reagent/Conditions:* Fuming sulfuric acid or oleum.
- Benzene undergoes electrophilic aromatic sulfonation to introduce the SO₃H group.
2. **Friedel-Crafts Acylation:**
- *Reagent/Conditions:* Acetyl chloride (CH₃COCl) and AlCl₃ catalyst.
- This reaction introduces the acyl group onto the aromatic ring, forming the acetylbenzenesulfonic acid.
**Key Considerations:**
- **Order of Reactions:** The sulfonation typically occurs first due to the directing effects on aromatic substitution.
- **Reaction Conditions:** Control of temperature and choice of catalyst is crucial for successful synthesis.
- **Orientation and Directing Effects:** Both groups direct further substitutions to ortho/para positions, making selective substitutions necessary.
**Conclusion:**
By following these steps with careful control over conditions and reagents, the target compound, acetylbenzenesulfonic acid, can be synthesized efficiently from benzene. This exercise demonstrates important concepts in electrophilic aromatic substitution reactions.
Expert Solution
Step 1
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY