How are equations 1 and 2 combined to that? Especially, where does the (n-1)/n comes from
How are equations 1 and 2 combined to that? Especially, where does the (n-1)/n comes from
Elements Of Electromagnetics
7th Edition
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Sadiku, Matthew N. O.
ChapterMA: Math Assessment
Section: Chapter Questions
Problem 1.1MA
Related questions
Question
100%
How are equations 1 and 2 combined to that? Especially, where does the (n-1)/n comes from
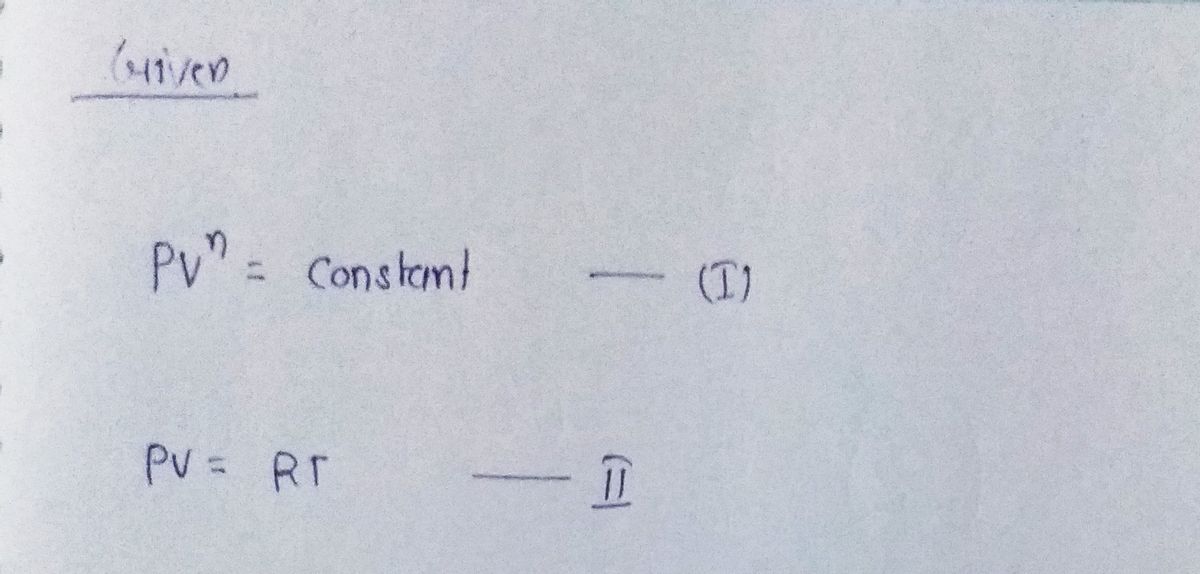
![**Polytropic Expansion Process**
**Expression of Polytropic Expansion Process:**
\[ PV^n = \text{Constant} \quad \text{(I)} \]
- **Variables:**
- \( P \) is the pressure
- \( V \) is the volume
- \( n \) is the polytropic index
**Expression of Ideal Gas Process:**
\[ PV = RT \quad \text{(II)} \]
- **Variables:**
- \( T \) is the temperature
- \( R \) is the gas constant
**Combining Equations (I) and (II):**
\[ T_2 = T_1 \left( \frac{P_1}{P_2} \right)^{(n-1)/n} \quad \text{(III)} \]
- **Variables:**
- \( P_1 \) is the initial absolute pressure
- \( P_2 \) is the final absolute pressure
- \( T_1 \) is the initial temperature
**Conclusion:**
Substitute \( 1200 \, \text{kPa} \) for \( P_1 \), \( 120 \, \text{kPa} \) for \( P_2 \), and \( 303 \, \text{K} \) for \( T_1 \) in Equation (III).
\[ T_2 = (303 \, \text{K}) \left( \frac{1200 \, \text{kPa}}{120 \, \text{kPa}} \right)^{0.2/1.2} \]
\[ T_2 = 445 \, \text{K} \]
Thus, the final temperature in a polytropic process is **445 K**.](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F629b4d07-b90d-4954-8498-0aa7a96e721c%2F124e0520-ee08-4f7e-b4b8-0b3d7b9a6360%2F4vo12x2_processed.png&w=3840&q=75)
Transcribed Image Text:**Polytropic Expansion Process**
**Expression of Polytropic Expansion Process:**
\[ PV^n = \text{Constant} \quad \text{(I)} \]
- **Variables:**
- \( P \) is the pressure
- \( V \) is the volume
- \( n \) is the polytropic index
**Expression of Ideal Gas Process:**
\[ PV = RT \quad \text{(II)} \]
- **Variables:**
- \( T \) is the temperature
- \( R \) is the gas constant
**Combining Equations (I) and (II):**
\[ T_2 = T_1 \left( \frac{P_1}{P_2} \right)^{(n-1)/n} \quad \text{(III)} \]
- **Variables:**
- \( P_1 \) is the initial absolute pressure
- \( P_2 \) is the final absolute pressure
- \( T_1 \) is the initial temperature
**Conclusion:**
Substitute \( 1200 \, \text{kPa} \) for \( P_1 \), \( 120 \, \text{kPa} \) for \( P_2 \), and \( 303 \, \text{K} \) for \( T_1 \) in Equation (III).
\[ T_2 = (303 \, \text{K}) \left( \frac{1200 \, \text{kPa}}{120 \, \text{kPa}} \right)^{0.2/1.2} \]
\[ T_2 = 445 \, \text{K} \]
Thus, the final temperature in a polytropic process is **445 K**.
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Elements Of Electromagnetics
Mechanical Engineering
ISBN:
9780190698614
Author:
Sadiku, Matthew N. O.
Publisher:
Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:
9780134319650
Author:
Russell C. Hibbeler
Publisher:
PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:
9781259822674
Author:
Yunus A. Cengel Dr., Michael A. Boles
Publisher:
McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:
9781118170519
Author:
Norman S. Nise
Publisher:
WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:
9781337093347
Author:
Barry J. Goodno, James M. Gere
Publisher:
Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:
9781118807330
Author:
James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:
WILEY