2.) One mole of a diatomic gas goes through a cycle in four steps: as shown in the V-T diagram. a) Draw the cycle in the P-V diagram. b) Calculate the work done on the gas and the heat added in each step. Explain! c) What is a possible use of such a cyclic process? Open-ended question. V [L] 1 4 2 1; +--- 300 350 not to scale T [K]
2.) One mole of a diatomic gas goes through a cycle in four steps: as shown in the V-T diagram. a) Draw the cycle in the P-V diagram. b) Calculate the work done on the gas and the heat added in each step. Explain! c) What is a possible use of such a cyclic process? Open-ended question. V [L] 1 4 2 1; +--- 300 350 not to scale T [K]
Related questions
Question
![2.) One mole of a diatomic gas goes through a cycle in four steps:
as shown in the V - T diagram.
a) Draw the cycle in the P - V diagram.
b) Calculate the work done on the gas and the heat added in each step.
Explain!
c) What is a possible use of such a cyclic process? Open-ended question.
V [L] t
4
2
21
1:
300
4
350
not to scale
T [K]](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F2a6f4774-f765-4df3-95cb-75f40ecafefb%2Fb0f078f4-c4ab-4d89-848b-baff6f17df57%2Favgzyyl_processed.jpeg&w=3840&q=75)
Transcribed Image Text:2.) One mole of a diatomic gas goes through a cycle in four steps:
as shown in the V - T diagram.
a) Draw the cycle in the P - V diagram.
b) Calculate the work done on the gas and the heat added in each step.
Explain!
c) What is a possible use of such a cyclic process? Open-ended question.
V [L] t
4
2
21
1:
300
4
350
not to scale
T [K]
Expert Solution
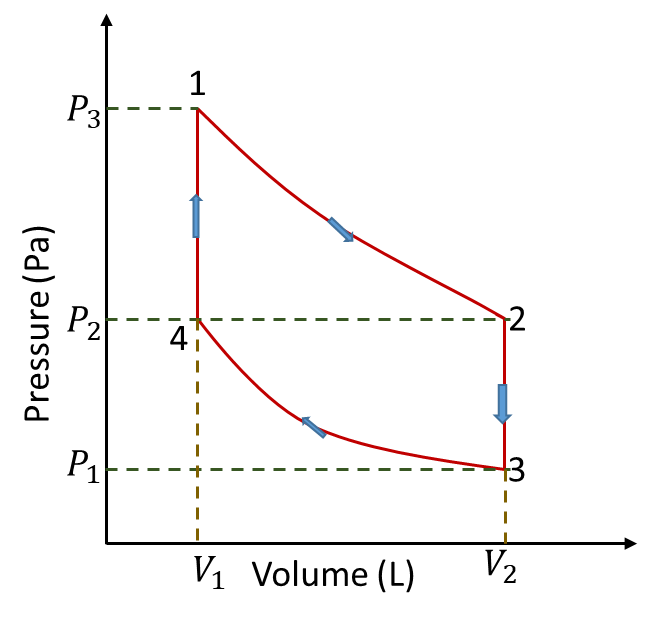
Part a)
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 1 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
This is definitely incorrect. The PV diagram shows the cycle traveling in the wrong direction. For example, per the VT diagram, the pressure from 4 to 1 must decrease, as it is cooling off with constant volume. In the solutions PV diagram, the pressure from 4 to 1 is increasing.
Solution