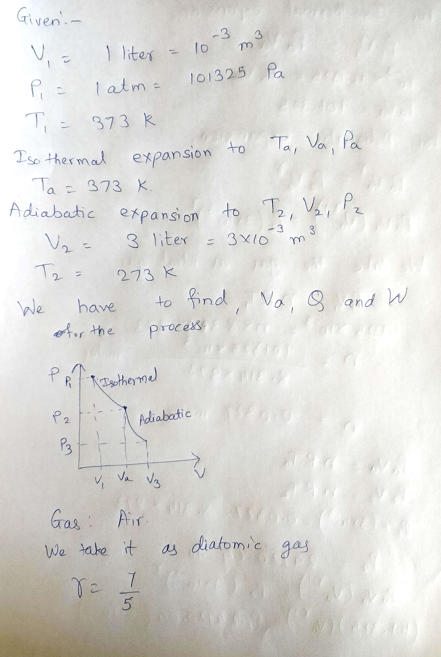
5) Air, initially at 373 K , 1 atm , and V,= 1 liter undergoes two successive reversible expansions- a reversible isothermal expansion to some intermediate volume V, followed by a reversible adiabatic expansion to a final volume V,=3 liters, cooling the gas to final temperature of 273 K. Calculate V,,Q, %3D and W, for the process. Assume ideal gas behavior.
5) Air, initially at 373 K , 1 atm , and V,= 1 liter undergoes two successive reversible expansions- a reversible isothermal expansion to some intermediate volume V, followed by a reversible adiabatic expansion to a final volume V,=3 liters, cooling the gas to final temperature of 273 K. Calculate V,,Q, %3D and W, for the process. Assume ideal gas behavior.
Related questions
Question

Transcribed Image Text:5) Air, initially at 373 K, 1 atm , and V, = 1 liter undergoes two successive reversible expansions – a
reversible isothermal expansion to some intermediate volume V, , followed by a reversible adiabatic
a
expansion to a final volume V, =3 liters, cooling the gas to final temperature of 273 K. Calculate V, Q,
and W, for the process. Assume ideal gas behavior.
Expert Solution
Step 1
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 4 images
