hic be sure your answer nas the correct sign! CH4 + 202 --> CO + 2H20 AH = -803 kJ/mol OR C H4+2 02 right arrow CO + 2H2 O Delta H = -803 kJ/mol Hint: Determine the limiting reagent. Do not type units with your answer. Type your answer to three significant figures. f this question is on an exam on your scrap paper report your answer to the proper umber of significant figures but still type three significant figures online. our Answer:
hic be sure your answer nas the correct sign! CH4 + 202 --> CO + 2H20 AH = -803 kJ/mol OR C H4+2 02 right arrow CO + 2H2 O Delta H = -803 kJ/mol Hint: Determine the limiting reagent. Do not type units with your answer. Type your answer to three significant figures. f this question is on an exam on your scrap paper report your answer to the proper umber of significant figures but still type three significant figures online. our Answer:
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
100%

Transcribed Image Text:**Problem Statement:**
How much heat (in kJ) is released when 170 grams of CH₄ is burned with 2000 grams of O₂? If the answer is exothermic, be sure your answer has the correct sign!
**Chemical Equation:**
CH₄ + 2O₂ → CO + 2H₂O ΔH = -803 kJ/mol
OR
C H₄ + 2 O₂ → CO + 2H₂O ΔH = -803 kJ/mol
**Instructions:**
- **Hint:** Determine the limiting reagent.
- **Note:** Do not type units with your answer.
- **Precision:** Type your answer to three significant figures.
**Additional Information:**
If this question is on an exam, report your answer on your scrap paper to the proper number of significant figures but still type three significant figures online.
**Your Answer:**
Expert Solution
Step 1
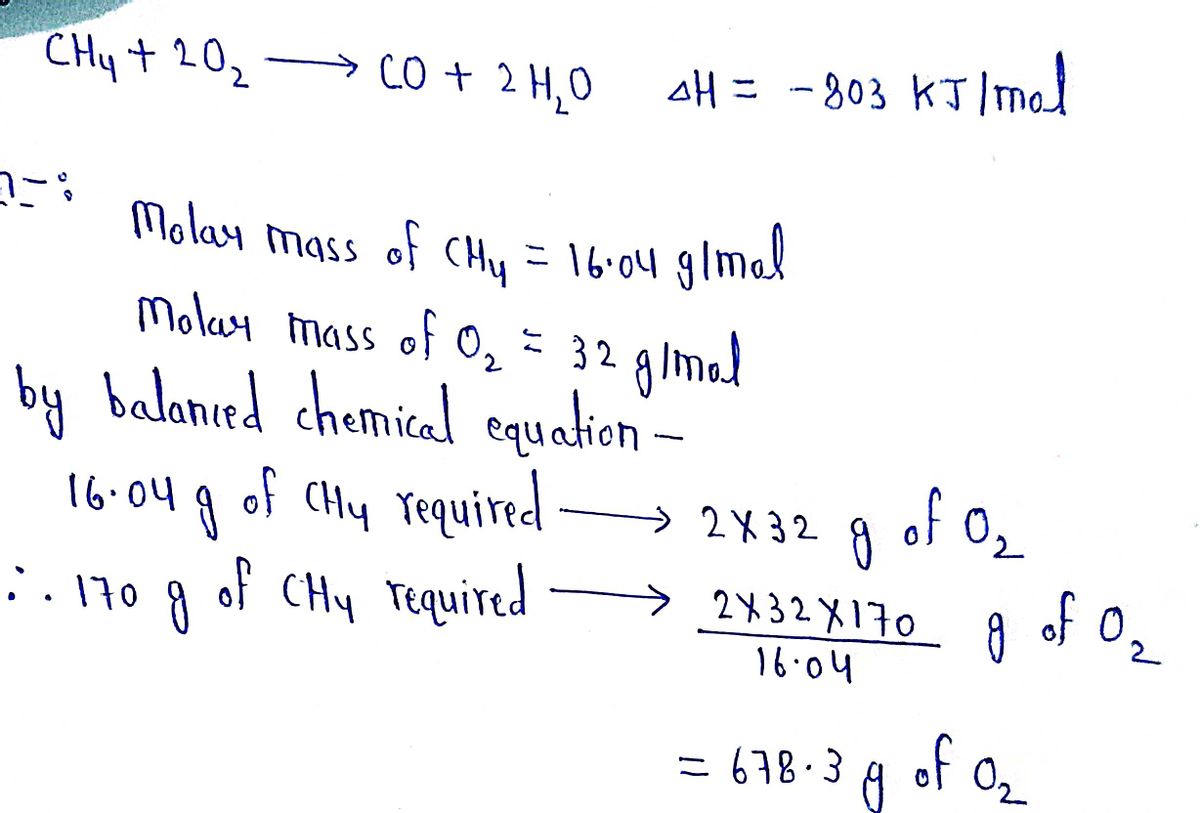
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY