Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
100%
I really need help analyzing and interpreting these spectra to find a chemical formula and compound structure!

Transcribed Image Text:210
200
190
84
-17094
170
160
150
140
130
120
110
100
f1 (ppm)
90
-77.16 CDCB
-64.05
13C NMR in CDCl3 (solvent is labeled)
-30.34
30
69 02-
2881
20
10
-10
-4500000
--4000000
-3500000
-3000000
-2500000
-2000000
-1500000
-1000000
-500000

Transcribed Image Text:10.0
2.5
4.0
9.0
3.5
8.5
T
3.0
8.0
7.26 CDC13
25
11 (ppm)
7.5
7.0
2.0
SS
6.5
15
B
10
5.5
-4.0x10²
--3.0x10²
-2.0x10²
10x10
00
5.0
11 (ppm)
4.5
4.07
---4.05
007
4.0
2.03
3.5
291
29 T
1.60
3.0
AST
AST
1.58
1.57
KT.
25
1.57
1.55
1.54
1.40
F000-2
20
-1.38
at
86 19
Forz
102
TTT T
15
1.36
F-2009
1.38
0.94
0.5
260
¹H NMR in CDC13 (solvent is labeled). Multiplicity for signals 1.25-1.75 is multiplet.
0.89
4.5×10²
40x10
-35x10
-3.0x10²
--25×10²
-20x10
15x10²
10x10
--50x10⁰
Loo
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
hello, thanks so much for this!! i also have an IR and Mass Spec spectra with this assignment. I figured out the IR to match these same signals and come up with this same compound, but I cant seem to figure out how to do the fragmentation on the Mass Spec. It is given then M+ = 116, but there's baRely a peak there
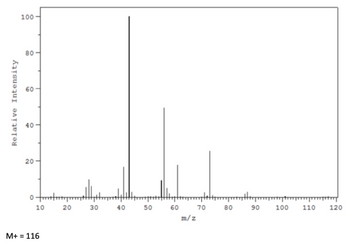
Transcribed Image Text:Relative Intensity
100
80
Ö
O
20
миниито
10
M+= 116
20
30 40
50
60
m/z
70
powqurubluquruqtangengungstag
100 110 120
80
90
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY