Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
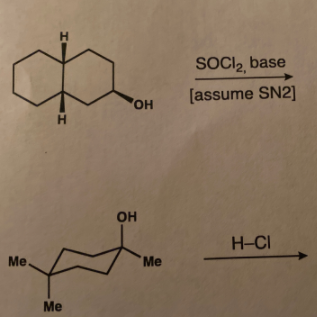
Draw the main product you expect from each reaction below. Pay attention to the stereochemistry.

Transcribed Image Text:The image shows a chemical reaction scheme involving the transformation of a cyclohexanol derivative.
**Reaction Details**:
1. **Reactant**: A cyclohexanol derivative featuring two methyl groups on the same carbon as the hydroxyl group (OH) and one methyl group on the adjacent carbon. The structure is a cyclohexane ring with the following substituents:
- Two methyl groups (Me) on the carbon with the hydroxyl group.
- An additional methyl group on the next carbon in the ring.
2. **Reagents**:
- The reaction uses thionyl chloride (SOCl₂) and a base.
- The reaction assumes an SN2 reaction mechanism.
3. **Product**:
- The product is another cyclohexanol derivative with two hydrogen atoms on different carbons and the hydroxyl group transferred.
**Side Product**:
- HCl is released during the reaction as a byproduct.
**Mechanism Overview**:
- The SN2 mechanism indicates a bimolecular nucleophilic substitution where the nucleophile attacks the substrate, leading to a transition state and inversion of configuration.
This reaction typically converts alcohols to alkyl chlorides, and in this context, it involves a cyclohexanol to a different structural isomer via interaction with thionyl chloride in the presence of a base.
Expert Solution
Step 1
The reactions given are,
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY