Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic steps. Be sure to account for all bond-breaking and bond-making steps. S H H HH Select to Add Arrows CH3CH₂OH 0: CH3CH₂ONa, heat :0: Na Ⓒ Select to Add Arrows H
Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic steps. Be sure to account for all bond-breaking and bond-making steps. S H H HH Select to Add Arrows CH3CH₂OH 0: CH3CH₂ONa, heat :0: Na Ⓒ Select to Add Arrows H
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:**Electron-Pushing Mechanism Illustration**
In organic chemistry, curved arrows are essential tools used to illustrate the flow of electrons. They depict how bonds are made and broken during chemical reactions.
**Instructions:**
Using the given starting and product structures, draw the curved electron-pushing arrows to reveal the mechanistic steps of the reaction below. Ensure you account for all bond-breaking and bond-making events.
**Reaction Details:**
1. **Starting Structure:**
- The initial molecule includes a six-membered ring (cyclohexane) with attached functional groups. A sodium ion (Na+) is nearby.
2. **Reagents:**
- CH₃CH₂ONa (sodium ethoxide)
- Heat is applied.
- CH₃CH₂OH (ethanol) is involved.
3. **Product Structure:**
- The resulting structure includes a carboxylate ion with an additional ethoxy group and ethanol by-product.
**Graphical Representation:**
- The diagram visualizes the transition between a cyclic molecule with a sodium ion and a carboxylate ion product. An intermediary step might involve removing a proton, facilitated by the curved electron-pushing arrows.
- **Select to Add Arrows**:
- When solving this problem, insert the curved arrows to show electron flow, highlighting significant transformations throughout the reaction mechanism. This often involves showing where lone pair electrons are moved to form new bonds or to break existing bonds.
Use these details to explore the mechanism of this reaction, focusing on electron movement to better understand how molecular changes occur.

Transcribed Image Text:The image depicts a chemical reaction scheme involving a reversible process.
**Description of the Reaction:**
1. **Reactants and Conditions:**
- Sodium ethoxide (\( \text{CH}_3\text{CH}_2\text{ONa} \))
- Heat
- Ethanol (\( \text{CH}_3\text{CH}_2\text{OH} \))
2. **Reaction Diagram (Central Box):**
- A skeletal structure showing a β-keto ester. The ester group is attached to a carbon-carbon double bond (enol form).
- An additional ethanol molecule is depicted separately.
- The structure has oxygen atoms marked with lone pairs, indicating the presence of carbonyl and hydroxyl groups.
3. **Reaction Arrows:**
- A large double-headed arrow signifies a reversible reaction, indicating equilibrium between reactants and products.
4. **Products:**
- It's suggested that the products include regenerated sodium ethoxide (\( \text{CH}_3\text{CH}_2\text{ONa} \)) and ethanol (\( \text{CH}_3\text{CH}_2\text{OH} \)), maintaining equilibrium with the reactant form.
**Overall Reaction Explanation:**
This schematic represents a mechanism potentially involving keto-enol tautomerism and ester formation, needing further clarification with specific arrows and intermediate steps for a detailed reaction path.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
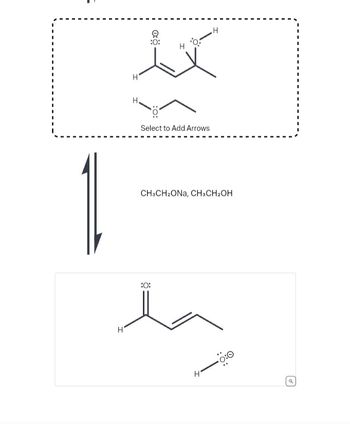
Transcribed Image Text:### Transcription for Educational Website:
#### Reaction Scheme
1. **Starting Materials:**
- Structure 1: The image shows a compound with a ketone group (C=O) and an allylic alcohol group.
- Structure 2: Ethanolate ion (CH₃CH₂O⁻).
Below the starting material is an arrow pointing downward labeled with the reagents:
- **Reagents:** CH₃CH₂ONa and CH₃CH₂OH.
2. **Reaction Product:**
- Structure 3: The product is a compound with an aldehyde group (CHO) and an allylic alcohol group.
- Silently, this transformation suggests a deprotonation step followed by rearrangement.
#### Explanation of Diagram:
- **Electron Flow:** The dashed box around the starting materials allows for the addition of arrow-pushing mechanisms to visualize the electron movement during the reaction. Select to add arrows to illustrate how the electron pairs rearrange as the reaction progresses.
- **Transition from Reactants to Products:**
- The reaction involves a base-catalyzed mechanism, where ethanolate acts as the nucleophile.
- The transformation typically involves the conversion of a carbonyl compound to a different functional group, guided by catalytic nucleophile action.
This diagram illustrates a common reaction mechanism in organic chemistry, likely involving alpha, beta-unsaturated carbonyl compounds and demonstrating nucleophilic addition or rearrangement processes.
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY