g to solve the following stoichiometry problem: "If 5.00 grams of propane burn completely, what carbon dioxide is produced at STP?" e following equation: C,H; + O, - Co, + H,0 van's work below, circle the errors and explain what change(s) you would make. Then solve the the "Show your work" box. I mol CsHy Imol CO, 22.4 L 44.11g CgH Imol CzHg mol CO
Atmospheric Pollution
In the atmosphere, the existence of large quantities of undesirable substances that could cause several health issues to living organisms and humans is atmospheric pollution. Air pollution is otherwise known to be atmospheric pollution. The presence of undesirable materials would also destruct the natural environment such as a change in climate, degradation of habitat, or depletion of ozone. Air pollution is generated by the natural processes and activities of humans.
Smokestack Scrubbers
Once we believe in the environment and remember the pollution-producing facets of it, we will consider the smoke-stacks scrubber to be a number of the worst offenders. Although this is often legally correct, smoke-stack scrubbers often have a big function in terms of keeping ground-level air pollutants- safe to breathe and assisting within the management of emissions.

In stoichiometric problems, first of all we have to balance the given reaction than start for further. In Ivan's calculation, moles of substances taken wrongly because Reaction was not balanced.
The balanced chemical reaction is as follow-
C3H8 + 5O2 ---> 3CO2 + 4H2O
Thus , the number of moles of Carbon dioxide wrongly taken -
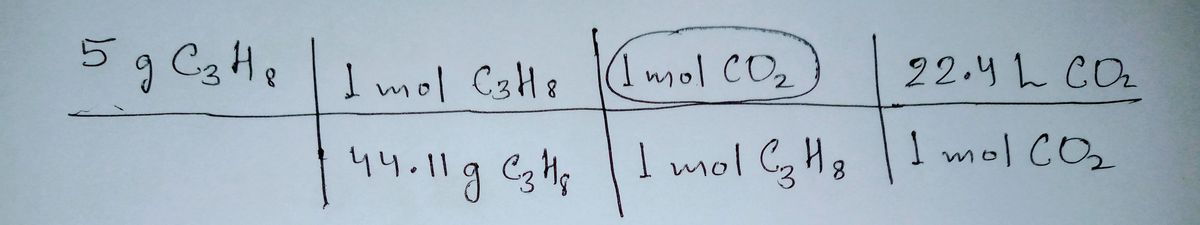
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images









