Examine the heating curve for water? Explain why the curve has two segments in which heat is added?
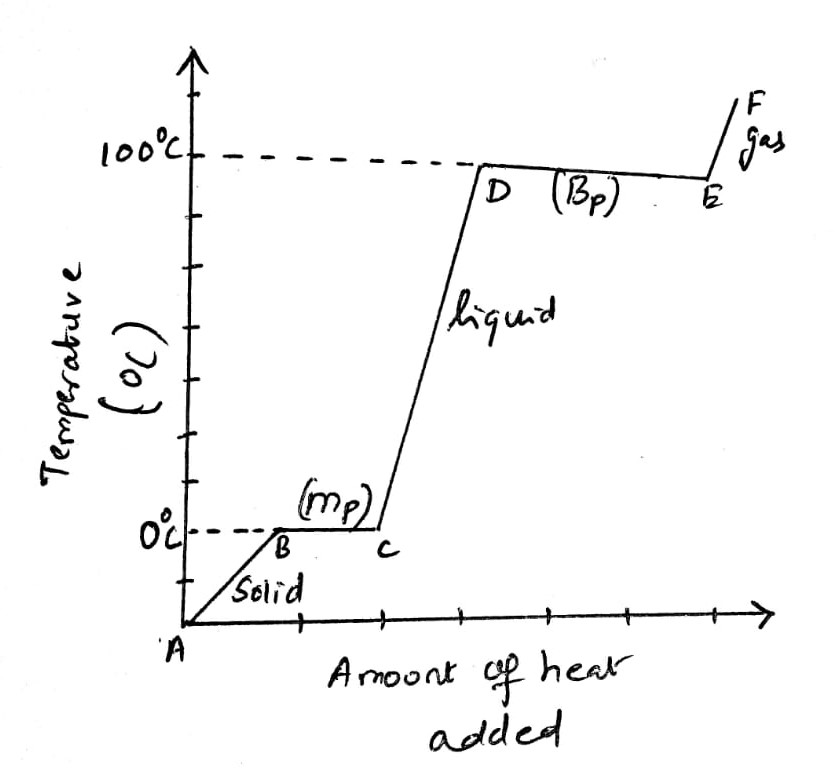
Water has hydrogens that are directly bonded to the oxygen atom. Water is capable of forming intermolecular hydrogen bonding and it enhances the physical properties such as melting and boiling point of water. The heating curve of water provides the relationship between the temperature change that leads to the phase change associated with the increase in the heat input to the system. The observation of the heating curve of water provides information about the exact amount of heat that should be applied for the phase changes (solid to liquid or liquid to vapor).
The heating curve of water as furnished as follows:
Explanation:
The heating curve has two segments due to the phase changes associated with water.
At 0°C, the point of B solid ice melts, and the horizontal line B to C denotes the phase change (solid to liquid). All the solid molecules are converted into the liquid phase maintaining the temperature to a constant value. Once all the solid melts, the temperature of the system increases since the water molecules absorb the heat (C to D).
At the point D (100°C), the second horizontal line is observed. At this point, all the liquid water molecules are converted into the vapor phase. The added heat is absorbed by the system to change the phase liquid to vapor. The line extends till E. The addition of a further amount of heat raises the temperature of the vapor phase.
Step by step
Solved in 3 steps with 1 images









