E MasteringCh x P Chemical eg X P Chemical eg X P Chemical eg X P Chemical ec X P Chemical ec X A session.masteringchemistry.com/myct/itemView?assignmentProblemID3D144082727&offset%3Dnext P Chemical ec X P Chemical e > CHE154-H Gen Chem II Bronikowski S20
E MasteringCh x P Chemical eg X P Chemical eg X P Chemical eg X P Chemical ec X P Chemical ec X A session.masteringchemistry.com/myct/itemView?assignmentProblemID3D144082727&offset%3Dnext P Chemical ec X P Chemical e > CHE154-H Gen Chem II Bronikowski S20
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question

Transcribed Image Text:E MasteringCh x P Chemical eg X
P Chemical eg X P Chemical eg X
P Chemical ec X
P Chemical ec X
A session.masteringchemistry.com/myct/itemView?assignmentProblemID3D144082727&offset%3Dnext
P Chemical ec X P Chemical e >
CHE154-H Gen Chem II Bronikowski S20
<CHE154 S20 Ch18 Sec4-6
Chapter 18 Algorithmic Question 60
Part A
A 25.0 mL sample of 0.150 M benzoic acid is titrated with a 0.150M NaOH solution. What is the pH at the equivalence point? The K of benzoic acid is 6.5 x 10.
11.20
4.20
9.80
7.20
8.53
Submit
Request Answer
vide Feedback
Expert Solution
Step 1
Given,
Volume of benzoic acid = 25.0 mL = 0.025 L (1 mL = 0.001 L)
Molarity of benzoic acid = 0.150 M = 0.150 mol/L
Molarity of NaOH = 0.150 M = 0.150 mol/L
Ka = 6.5 × 10-5
Step 2
Moles of benzoic acid can be calculated as :
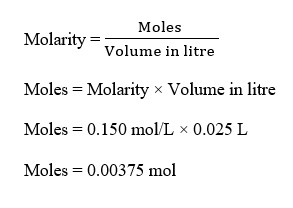
Step 3
The reaction of benzoic acid with strong base, NaOH can be written as :
C6H5COOH (aq) + OH- (aq) → C6H5COO- (aq) + H2O (l)
At equivalence point, the moles of benzoic acid will be equal to the moles of NaOH. Thus, we can calculate the volume of NaOH used as :
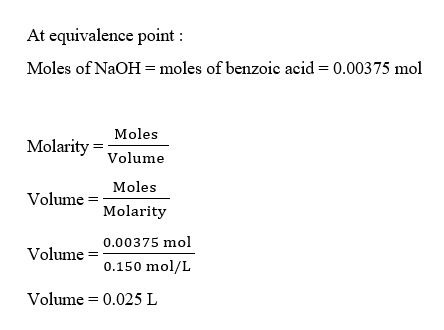
Step 4
At equivalence point, the concentration of C6H5COO- will be :
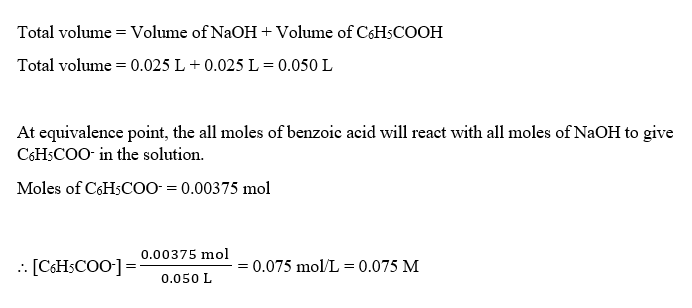
Step by step
Solved in 7 steps with 6 images

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY