Does a reaction occur when aqueous solutions of ammonium carbonate and iron(III) bromide are combined? O yes O no If a reaction does occur, write the net ionic equation. (Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds. Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank.) +
Does a reaction occur when aqueous solutions of ammonium carbonate and iron(III) bromide are combined? O yes O no If a reaction does occur, write the net ionic equation. (Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds. Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank.) +
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter8: Reactions In Aqueous Solutions
Section: Chapter Questions
Problem 5STP
Related questions
Question
100%
9 and 14

Transcribed Image Text:## Reaction Evaluation and Net Ionic Equation
### Problem Statement:
**Question:** Does a reaction occur when aqueous solutions of ammonium carbonate and iron(III) bromide are combined?
- **Options:**
- Yes (selected)
- No
**Instruction:** If a reaction does occur, write the net ionic equation.
*Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.*
### Diagram Explanation:
This section includes a diagram for inputting the reactants and the net ionic equation. The diagram consists of two boxes for the reactants, an arrow indicating a chemical reaction, and one box for the product(s).
- **Reactants:** Two boxes with a plus sign between them represent the reactants that are combined.
- **Arrow:** Indicates the direction of the reaction leading to product(s).
- **Products:** One box to write the net ionic equation for the product(s) formed.
This setup assists in determining whether a reaction occurs and facilitates the writing of the net ionic equation based on solubility rules.

Transcribed Image Text:**Transcription for Educational Website:**
---
**Instructions:**
- Be sure to specify states such as (aq) or (s).
- If a box is not needed, leave it blank.
- If no reaction occurs, leave all boxes blank and click on "Submit".
**Problem:**
Write a net ionic equation for the reaction that occurs when aqueous solutions of **hydrobromic acid** and **barium hydroxide** are combined.
(*Use H⁺ instead of H₃O⁺.*)
**Equation Layout:**
(Reactant 1) + (Reactant 2) → (Product 1) + (Product 2)
---
**Guidance:**
To solve this problem, consider the dissociation of hydrobromic acid (HBr) and barium hydroxide (Ba(OH)₂) in water and then identify the ions that participate in the reaction to form the net ionic equation. Remember to include states of matter for each ion and compound.
Expert Solution
Step 1
First we have to write the complete equation using the solubility rules.
Then we can write complete ionic equation and hence net ionic equation.
Solubility rules used here are given as :
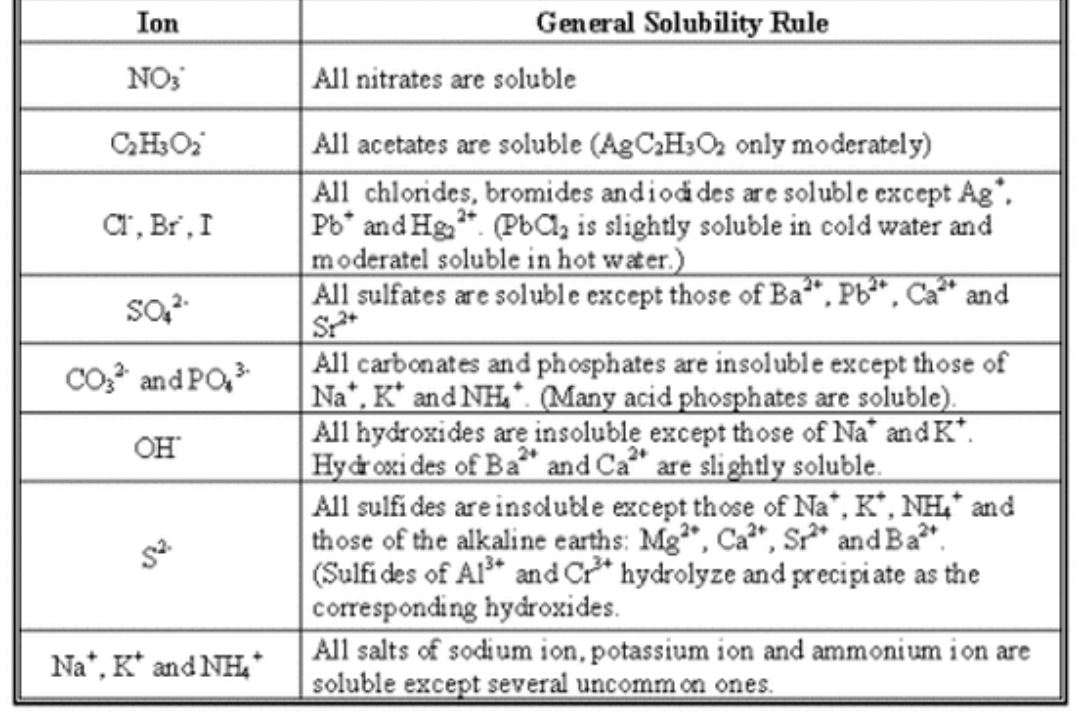
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning