Do you believe it is easier to measure atomic masses or nuclear masses? Explain how you could experimentally measure both for 2H. What about for 40Ca?
Do you believe it is easier to measure atomic masses or nuclear masses? Explain how you could experimentally measure both for 2H. What about for 40Ca?
Related questions
Question
Do you believe it is easier to measure atomic masses or nuclear masses? Explain how you could experimentally measure both for 2H. What about for 40Ca?
Expert Solution
Step 1
It is easier to measure nuclear mass of the atom than atomic mass, because atomic mass can only be measured when atom is in atomic state whereas nuclear mass can be measured in atomic state as well as in ionic state.
Step 2
H2 atom has :
number of proton =1 , mp=mass of proton
number of neutron=1 , mn=mass of neutron
number of electron=1 , me=mass of electron
Atomic mass =1me+1mp+1mn
Nuclear mass = 1mp+1mn
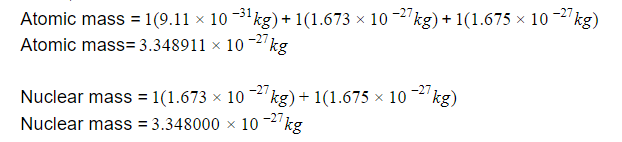
Step by step
Solved in 3 steps with 2 images
