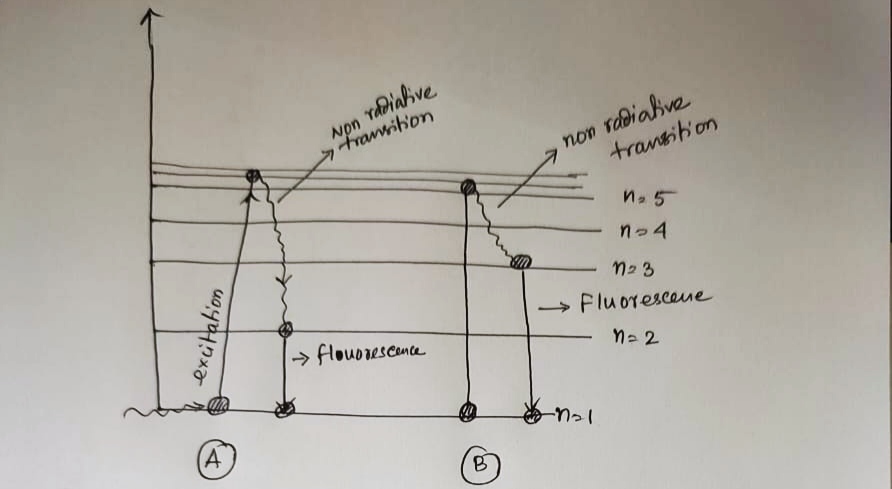
diagram above, draw and label a set of energy transitions that show the process of fluorescence.
Q: Block A is a steel block at a temperature of 500 K and Block B is an identical block but at a…
A: Temperature of A =500K Temperature of B =1000K
Q: The magnetic vector potential radiated from a dipole antenna is calculated to be A era, where K is…
A: To find the radial component of the magnetic vector potential in spherical polar coordinates, we can…
Q: The frequency of violet light is about twice that of red light. Which has more energy - a photon of…
A: Given, Frequency of violet light and it is twice of red light.
Q: 6. The lithium surface in a photoelectric cell will emit electrons when the incident light is blue.…
A:
Q: a) What does the shift in the wavelength tell us about the motion of the object? b) A second star is…
A: this question is about stars are moving away or towards to earth . wavelength is shifted means stars…
Q: Problems 1. When hydrogen is energized by a strong electric field or incident ultraviolet light, the…
A: In this question we are given that When hydrogen is energized by a strong electric field or incident…
Q: Explain how it is possible to identify the atomic number of an element from the wavelengths of the…
A:
Q: (a) Calculate the ionisation energy, in eV. for the hydrogen atom. (b) Calculate the frequency of a…
A: The energy levels of the Hydrogen atom
Q: 1. When hydrogen is energized by a strong electric field or incident ultraviolet light, the gas…
A:
Q: In a non-destructive test, for a 100-keV x-ray beam, calculate the fraction of beam intensity…
A:
Q: TEST YOUR UNDERSTANDING OF SECTION 38.2 In the apparatus shown in Fig. 38.7, suppose yau increase…
A: Given: we are increasing no of electrons emitted from cathode per second and keeping potential…
Q: I. The shadow cast by a small bead placed in laser light has a bright spot in the middle II.…
A: Light has dual nature, depending on situation light can interact as wave as well as particle nature.
Q: The type of light that can be emitted when one electron orbiting a hydrogen atom drops down in its…
A: Concept used: Photon is emitted from excited electron falls back to its state.
Q: Which of the following statements is NOT true regarding electric charges? a. A neutral atom contains…
A: Correct answer is Option (d) i .e. S .I unit of electric charge is Ampere . Reason :…
Q: 2.) The (net) current in a solar cell can only go in one direction. Explain which direction is…
A: Solar cell.
Q: Pulsed lasers have many applications, but are very complicated to construct. One problem is…
A:
Q: What is the energy of the photon an electron in hydrogen must absorb to jump from the 1st to 5th…
A: Electron in hydrogen atom makes a transition from 1st to 5th energy state. Find: Energy absorbed by…
Q: Calculate the energies predicted in the following transitions for the hydrogen atom. Show all of…
A: Given: Planck's constant, h=6.626×10-34JsSpeed of light, c=3×108m/sWavelength of violet,…
Q: Choose the steps below in the appropriate order to explain why the sky is blue. O Light that is…
A: Part A White light with all colors is emitted by the sun White light interacts with molecules in…
Q: 13.50eV 6.00ev 1.50ev Which equation would you use to calculate the frequencies of emission using…
A:
PLEASE ANSWER ON THE EXACT SAME PHOTO, LAST TIME YOU USED A DIFFERENT PHOTO. On the diagram above, draw and label a set of energy transitions that show the process of fluorescence.
![0 eV
-0.54 eV
-0.85 eV
-1.51 eV
-3.40 eV
-13.6 eV
n=5
n=4
n=3
n=2
n=1
[This diagram is not drawn to scale.]](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F9f301fe7-a9d6-4ec9-ae08-d8d4471271f3%2Fa60dcdd5-3d5b-4423-9d64-61f137f939d6%2F2nbe90c_processed.png&w=3840&q=75)
Step by step
Solved in 3 steps with 2 images

- Please write it in detailed and clean solution.The emission spectrum for the atoms of a gas is shown. Which of the energy level diagrams below corresponds to this spectrum? The energy levels shown above in selection a) The energy levels shown above in selection b) The energy levels shown above in selection c) d)Please answer all parts with lots of explanation
- Titanium (ϕ = 6.94 ✕ 10−19 J) and silicon (ϕ = 7.24 ✕ 10−19 J) surfaces are irradiated with UV radiation with a wavelength of 250 nm. Which surface emits electrons with the longer wavelength? What is the wavelength of the electrons emitted by the titanium surface? Show your work.The frequency of Helium Neon laser light is 4.5x1014 Hz. Calculate the energy of the photon in eV, Show your workUse the following information to answer the next question The different colours seen in exploding fireworks are produced using different elements. Element Predominant colour Strontium Red Barium Green Copper Blue-green Sodium Yellow-orange 25. Given the information above, the element that emits the lowest energy photon of visible light is: O barium O copper O strontium O sodium Your reasoning: