Question 5
Determine the hybridization of the metal centre in the complexes below using Ligand Field Theory.
1. [RhCl3(OH)3]3-
2. [Cr(en)2(PMe3)2]
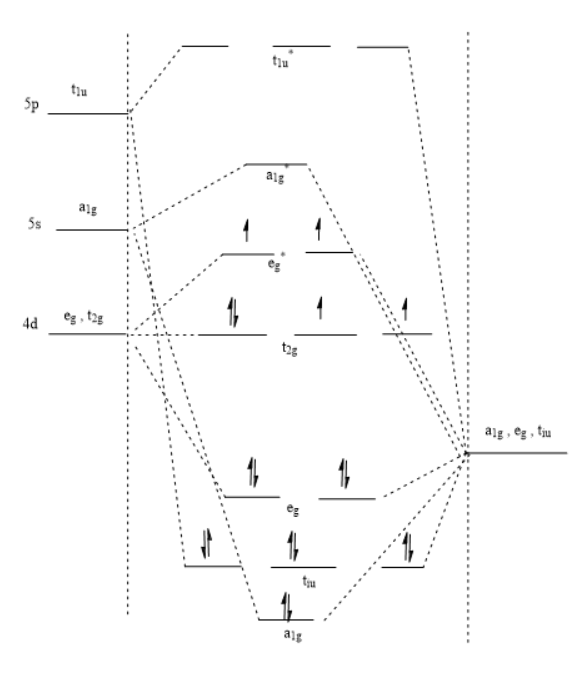
Ligand field theory (LFT) describes the bonding, orbital arrangement, and other characteristics of coordination complex. It represents an application of molecular orbital theory to transition metal complexes. A transition metal ion has nine valence atomic orbital - consisting of five nd, one (n+1)s, and three (n+1)p orbitals. These orbitals are of appropriate energy to form bonding interaction with ligands. The LFT analysis is highly dependent on the geometry of the complex, but most explanations begin by describing octahedral complexes, where six ligands coordinate to the metal. Other complexes can be described by reference to crystal field theory.
1. In complex , Rh have +3 oxidation state having configuration , thus give 6 electrons. Ligands also contribute 12 electrons , thus 18 electrons total . Also Cl and OH are weak ligands thus no pairing occurs. So the hybridization of the complex is and orbital diagram is :
Step by step
Solved in 3 steps with 2 images









